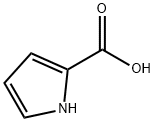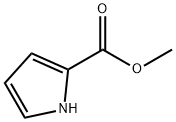
Methyl 2-pyrrolecarboxylate synthesis
- Product Name:Methyl 2-pyrrolecarboxylate
- CAS Number:1193-62-0
- Molecular formula:C6H7NO2
- Molecular Weight:125.13
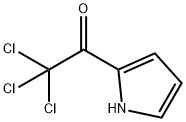
35302-72-8
178 suppliers
$10.00/5g

124-41-4
708 suppliers
$12.00/25g

1193-62-0
229 suppliers
$5.00/1g
Yield:1193-62-0 95%
Reaction Conditions:
in methanol at 20; for 2 h;
Steps:
Methyl-1H-pyrrol-2-carboxylate (5)
Sodium(0.223 g, 9.7 mmol) was dissolved in dry MeOH(64 mL) at room temperature. To the resulting solution,(2-trichloroacetyl)-1H-pyrrole (14.700 g, 69.2 mmol)was added in six portions under stirring for 30 min.The reaction mixture was stirred for 2 h at room temperature(control by TLC). The solvent was evaporated,and the residue was diluted with Et2O (80 mL). To the solution, 3 M HCl (5 mL) was added, theorganic phase was separated, and the aqueous phasewas extracted with Et2O (3 × 15 mL). The combinedorganic phase was successively washed with a saturatedaqueous NaHCO3 solution and a saturatedaqueous NaCl solution and dried over Na2SO4. Thedrying agent was filtered off, and the solvent was evaporatedunder low pressure. At the next stage, the productwas used without additional purification. Ether (5)was obtained as white crystals. Yield 8.215 g (95%); Rf0.31 (PE-EtOAc 5 : 1); mp 73.0°C. The spectral characteristicscoincided with those reported in the literature[6]. 1H NMR (CDCl3): 3.86 (s, 3H), 6.26-6.28(m, 1H), 6.91-6.93 (m, 1H), 6.95-6.97 (m, 1H), 9.27(m, 1 H).
References:
Fedarkevich, A. N.;Sharko, O. L.;Shmanai, V. V. [Russian Journal of Bioorganic Chemistry,2020,vol. 46,# 2,p. 187 - 198]

67-56-1
771 suppliers
$9.00/25ml

35302-72-8
178 suppliers
$10.00/5g

1193-62-0
229 suppliers
$5.00/1g

2133-40-6
442 suppliers
$8.00/5g

1193-62-0
229 suppliers
$5.00/1g

35302-72-8
178 suppliers
$10.00/5g

1193-62-0
229 suppliers
$5.00/1g