
(S)-N-(2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)quinoline-4-carboxamide synthesis
- Product Name:(S)-N-(2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)quinoline-4-carboxamide
- CAS Number:1448440-52-5
- Molecular formula:C17H14F2N4O2
- Molecular Weight:344.32
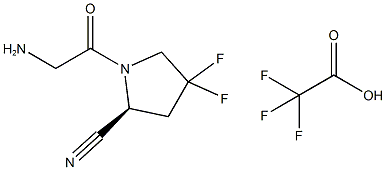
1448440-40-1
16 suppliers
inquiry

486-74-8
245 suppliers
$15.00/250mg

1448440-52-5
59 suppliers
inquiry
Yield: 45%
Reaction Conditions:
Stage #1:4-quinolinic acid with benzotriazol-1-ol;O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate;N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide for 0.25 h;
Stage #2:(S)-1-(2-aminoacetyl)-4,4-difluoropyrrolidin-2-carbonitrile 2,2,2-trifluoroacetate in N,N-dimethyl-formamide at 20;
Steps:
1
2.2 Synthesis of final products of formula (I), via intermediates (D) and (E), as defined in Scheme 1. -(2-(2-cyano-4,4-difluoropyrrolidin-1-yl)-2-oxoethyl)quinoline-4-carboxamide Commercially available quinoline-4-carboxylic acid (0.065 g, 0.377 mmol) was dissolved in 7 ml of DMF, A/-ethyl-/V-isopropylpropan-2-amine (0.210 ml, 1 .168 mmol), HOBT (0.058 g, 0.377 mmol) and TBTU (0.121 g, 0.377mmol) were added. After 15 min a solution of (S)-1 -(2-aminoacetyl)-4,4- difluoropyrrolidine-2-carbonitrile trifluoroacetate (0.085g, 0.377mmol) (prepared as described under A.2 of the experimental part) in DMF was added. The mixture was stirred overnight at room - - temperature. The volatiles were evaporated, the residue was dissolved in ethyl acetate and washed with 1 N citric acid, saturated sodium bicarbonate and brine. The solution was dried over sodium sulfate, filtrated and evaporated. It was purified using column chromatography (1-4 hexane- ethyl acetate). Yield: 64mg, 45% 1H NMR (400 MHz, CDCI3): (8.5/1.5 mixture of trans/cis amide rotamers) δ 2.72 - 2.83 (m, 2H), 3.91 -4.07 (m, 2H), 4.21 (dd, J= 17.4, 4.2 Hz, 0.85H), 4.33 (dd, J= 17.4, 4.3 Hz, 0.15H), 4.39 (dd, J= 17.4, 5.6 Hz, 0.85H), 4.70 (dd, J= 17.4, 5.7 Hz, 0.15H), 4.92-4.99 (m, 0.85H), 5.15 (d, J = 9 Hz, 0.15H), 7.30 (s, 1 H), 7.49 (dd, J= 10.11 , 4.30 Hz, 1 H), 7.60 (dd, J = 11.22, 4.11 Hz, 1 H), 7.74 (t, J= 7.69 Hz, 1H), 8.12 (d, J= 8.42 Hz, 1H), 8.23 (t, J= 9.99 Hz, 1H), 8.96 - 8.86 (m, 1H). MS (ESI) m/z 345.0 [M+1]+ LC-MS (l-B) Rt10.8 min, m/z 345.0 [M+H]+ (98%).
References:
UNIVERSITEIT ANTWERPEN;FOX CHASE CANCER CENTER;JANSEN, Koen;DE MEESTER, Ingrid;HEIRBAUT, Leen;CHENG, Jonathan D;JOOSSENS, Jurgen;AUGUSTYNS, Koen;VAN DER VEKEN, Pieter WO2013/107820, 2013, A1 Location in patent:Page/Page column 47; 48
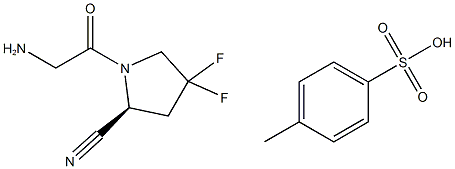
1584652-36-7
9 suppliers
inquiry

486-74-8
245 suppliers
$15.00/250mg

1448440-52-5
59 suppliers
inquiry
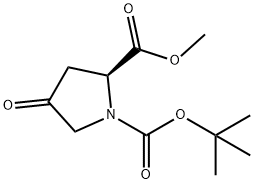
102195-80-2
234 suppliers
$5.00/250mg

1448440-52-5
59 suppliers
inquiry
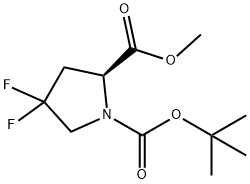
203866-17-5
135 suppliers
$13.00/100mg

1448440-52-5
59 suppliers
inquiry
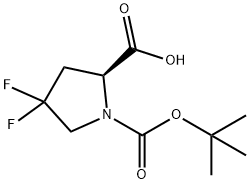
203866-15-3
174 suppliers
$15.00/250mg

1448440-52-5
59 suppliers
inquiry