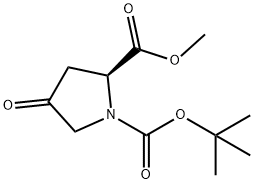
N-Boc-4-oxo-L-Proline methyl ester synthesis
- Product Name:N-Boc-4-oxo-L-Proline methyl ester
- CAS Number:102195-80-2
- Molecular formula:C11H17NO5
- Molecular Weight:243.26
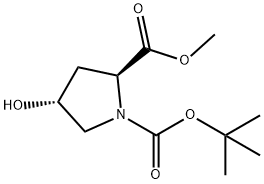
74844-91-0
405 suppliers
$5.00/5g

102195-80-2
234 suppliers
$5.00/250mg
Yield:102195-80-2 98.17%
Reaction Conditions:
with 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical;trichloroisocyanuric acid in dichloromethane at 0 - 20; for 0.5 h;
Steps:
43.A Step A:
To a solution of N-Boc-trans-4-hydroxyl-L-methyl prolinate (114.00 g, 464.79 mmol) in dichloromethane (1.2 L) was added trichloroisocyanuric acid(113.42 g, 488.03 mmol) and then TEMPO (1.46 g, 9.30 mmol) was added at 0 °C.
After stirring for 0.5 h at 10-20 °C, the mixture was filtered with kieselguhr.
The dichloromethane phase was washed with sat. aq K2CO3 (1000 mL) twice, then with sat.aq NaCl (800 mL), dried over anhydrous Na2SO4, filtered and the filtrate was concentrated in vacuo to give N-Boc- 4-oxo-L-methyl prolinate (111.00 g, 456.30 mmol, 98.17%).
1H NMR (CDCl3, 400 MHz): δ 4.81-4.64 (m, 1H), 3.84 (d, J = 7.8 Hz, 2H), 3.73 (s, 3H), 2.99-2.84 (m, 1H), 2.55 (d, J = 20.0 Hz, 1H), 1.43 (d, J = 8.0 Hz, 9H).
References:
Medshine Discovery Inc.;SUN, Fei;DING, Charles Z.;CAI, Zhe;QIAN, Wenyuan;HU, Guoping;LI, Jian;CHEN, Shuhui EP3450430, 2019, A1 Location in patent:Paragraph 0272; 0273

796095-60-8
1 suppliers
inquiry

102195-80-2
234 suppliers
$5.00/250mg

79-37-8
465 suppliers
$17.67/10gm:

796095-60-8
1 suppliers
inquiry
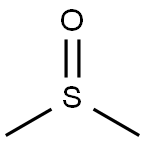
67-68-5
1211 suppliers
$15.00/100g

102195-80-2
234 suppliers
$5.00/250mg
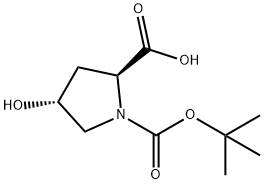
13726-69-7
428 suppliers
$5.00/1g
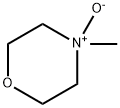
7529-22-8
382 suppliers
$5.00/1g

102195-80-2
234 suppliers
$5.00/250mg

24424-99-5
820 suppliers
$13.50/25G

102195-80-2
234 suppliers
$5.00/250mg