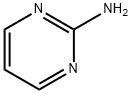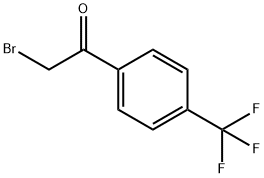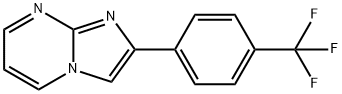
2-(4-(trifluoromethyl)phenyl)imidazo[1,2-a]pyrimidine synthesis
- Product Name:2-(4-(trifluoromethyl)phenyl)imidazo[1,2-a]pyrimidine
- CAS Number:1449488-26-9
- Molecular formula:C13H8F3N3
- Molecular Weight:263.22
Yield:1449488-26-9 66%
Reaction Conditions:
with sodium hydrogencarbonate in ethanol; for 8 h;Reflux;
Steps:
3
General procedure: To a solution of 2-aminoheteroarene (10mmol) in ethanol (50mL), phenacyl bromide (10mmol) and NaHCO3 (30mmol) were added at rt and the reaction mixture was refluxed for 8h. On removal of solvent, the residue was dissolved with ethyl acetate (100mL) and washed with water (50mL), brine (50mL), and dried over sodium sulfate. The solvent was evaporated to give crude product, which was purified by combiflash chromatography (1-20% EtOAc/PE) to give the desired product 2-(4-(trifluoromethyl)phenyl)imidazo[1,2-a]pyrimidine (4b)
Isolated yield 66%; Rf (1:9 MeOH/chloroform) 0.53; off white solid: mp 233-235 °C; 1H NMR (300 MHz, CDCl3) δ (ppm): 7.46 (dd, J=4.2, 6.6 Hz, 1H), 7.94 (d, J=8.1 Hz, 2H), 8.24 (d, J=8.1 Hz, 2H), 8.78 (s, 1H), 8.88 (dd, J=1.6, 4.2 Hz, 1H), 9.22 (dd, J=1.8 Hz, 4.2 Hz, 1H); 13C NMR (75 MHz, CDCl3) δ (ppm): 111.1, 113.4, 126.7 (q), 127.3 (*2C), 132.7, 137.1, 137.9 (*2C), 145.9, 156.6; HRMS (ESI): m/z [M+H]+ calcd for C13H9F3N3: 264.0743, found 264.0746; HPLC: 96.3%.
References:
Patil, Shashikant M.;Kulkarni, Sarang;Mascarenhas, Malcolm;Sharma, Rajiv;Roopan, S. Mohana;Roychowdhury, Abhijit [Tetrahedron,2013,vol. 69,# 38,p. 8255 - 8262]