
Pyrimidine, 2-chloro-5-fluoro-4-(2-fluoro-4-pyridinyl)- synthesis
- Product Name:Pyrimidine, 2-chloro-5-fluoro-4-(2-fluoro-4-pyridinyl)-
- CAS Number:1453851-91-6
- Molecular formula:C9H4ClF2N3
- Molecular Weight:227.6
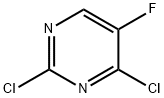
2927-71-1
407 suppliers
$6.00/5g
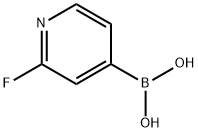
401815-98-3
243 suppliers
$10.00/250mg

1453851-91-6
3 suppliers
inquiry
Yield:1453851-91-6 86%
Reaction Conditions:
with dichloro(1,1'-bis(diphenylphosphanyl)ferrocene)palladium(II)*CH2Cl2;sodium carbonate in 1,4-dioxane;water at 80; for 1.5 h;Inert atmosphere;Sealed tube;Suzuki Coupling;
Steps:
A
(002651 Step A: Sodium carbonate (1.3 g, 13 mmol) to 2-fluoropyridin-4-ylboronic acid (0.71 g, 5.0 mmol) and 2,4-dichloro-5-fluoropyrimidine (0.70 g, 4.2 mmol) in dioxane/water (17 mL, 4:1), and the mixture was purged with nitrogen. PdC12(dppf)*DCM (0.17 g, 0.21 mmol) was added to the mixture, and the sealed vial was heated at 80°C. After 1.5 hours, the cooled reaction mixture was partitioned between water and EtOAc. The EtOAc was washed with brine, dried over MgSO4, filtered, and evaporated to yield a crude product (1.7 g) as an oil. The crude product was absorbed on silica gel and chromatographed on a 50 g Biotage SNAP column with 2:1 hexane/EtOAc. Fractions 14-17 contained 2-chloro-5- fluoro-4-(2-fluoropyridin-4-yl)pyrimidine (0.82 g, 3.6 mmol, 86% yield).
References:
WO2013/130976,2013,A1 Location in patent:Paragraph 00265