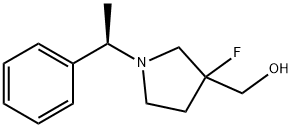
{3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidin-3-yl}methanol synthesis
- Product Name:{3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidin-3-yl}methanol
- CAS Number:1461698-16-7
- Molecular formula:C13H18FNO
- Molecular Weight:223.29
![methyl 3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidine-3-carboxylate](/CAS/20210111/GIF/1461698-11-2.gif)
1461698-11-2
4 suppliers
inquiry
![{3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidin-3-yl}methanol](/CAS/20200331/GIF/1461698-16-7.gif)
1461698-16-7
4 suppliers
inquiry
Yield:1461698-16-7 88%
Reaction Conditions:
with lithium aluminium tetrahydride in tetrahydrofuran at 0 - 20; for 4 h;Inert atmosphere;
Steps:
4.6. {3-Fluoro-1-[(1R)-1-phenylethyl]pyrrolidin-3-yl}methanol(14)
In a two-necked flask were charged 150 mL of freshly distilledTHF and LiAlH4 (3.1 g, 0.082 mol, 1.1 equiv). The reaction mixturewas cooled to 0 C and the solution of 13 (19.0 g, 0.075 mol, 1 equiv)in THF (150 mL) was carefully added dropwise. Then the reactionmixture was warmed to room temperature and stirred for an additional4 h. After that the reaction mixture was cooled to 0 C and20 mL of THF/water9/1 was carefully added. After stirring for10 min the mixture was filtered through a Buchner funnel and thesolid was washed twice with 150 mL of CH2Cl2. The combined organicphase was dried over Na2SO4 and evaporated under vacuumto give 14 (14.8 g, 0.066 mol, 88% yield) as a yellow oil.1H NMR (500 MHz; CDCl3, Me4Si; 2 diastereoisomers are present)d: 1.38e1.41 (m, 3H, CHCH3), 1.70 (br s, 1H), 1.95e2.14 (m, 3H),2.65e2.87 (m, 3H), 3.26e3.32 (m, 1H), 3.65e3.75 (m, 2H),7.24e7.35 (m, 5H, C6H5).13C NMR (125 MHz; CDCl3; Me4Si; 2 diastereoisomers arepresent), d: 22.7, 33.8 (d, J9 Hz), 51.3, 60.6, 65.4 (s), 66.9 (d,J10 Hz), 103.8 (d, JCF178 Hz, CF), 127.13 (s, C6H5), 127.17 (s, C6H5),128.4 (s, C6H5), 144.6 (C6H5).19F NMR (477 MHz; C6F6;Me4Si; 2 diastereoisomers are present)d: 150.5 (149.8) (m, J23 Hz).Anal. Calcd for C13H18FNO: C, 69.93; H, 8.13; N, 6.27. Found: C,69.88; H, 8.19; N, 6.32.LCeMS: 224 (M).[a]D20 12.52 (c 0.78 g/100 mL, MeOH).
References:
Yarmolchuk, Vladimir S.;Mykhalchuk, Vladimir L.;Mykhailiuk, Pavel K. [Tetrahedron,2014,vol. 70,# 18,p. 3011 - 3017]
133407-38-2
54 suppliers
$45.00/50mg
![{3-fluoro-1-[(1R)-1-phenylethyl]pyrrolidin-3-yl}methanol](/CAS/20200331/GIF/1461698-16-7.gif)
1461698-16-7
4 suppliers
inquiry