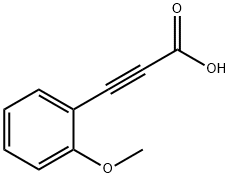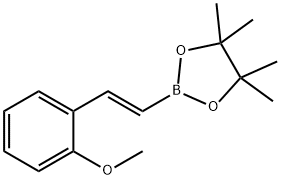
2-Methoxy-trans-beta-styrylboronic acid pinacol ester synthesis
- Product Name:2-Methoxy-trans-beta-styrylboronic acid pinacol ester
- CAS Number:149777-81-1
- Molecular formula:C15H21BO3
- Molecular Weight:260.14
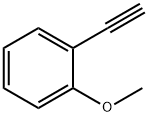
767-91-9
120 suppliers
$10.00/250mg
25015-63-8
223 suppliers
$22.39/5G

149777-81-1
25 suppliers
inquiry
Yield:149777-81-1 81%
Reaction Conditions:
with C28H40CuNO in acetonitrile at 20; for 2 h;Schlenk technique;Inert atmosphere;
Steps:
A General Protocol
General procedure: To a flame dried Schlenk was added Et2CAACCuOPh (2.5 mol %) and a magnetic stir bar under an Ar atmosphere. 0.097 mL freshly distilled MeCN was added to fully dissolve catalyst and yield a 2.3 ± 0.1 M solution depending on the nature of the alkyne. Alkyne (0.56 mmol, 1 eq.) was added followed immediately by pinacolborane (0.57 mmol, 1.025 eq). The resulting solution is stirred at room temperature for 2 hours. After this time, the volatiles were evaporated under vacuum. 10 mL pentane was added to residue. This solution was passed through a pad of silica (5 cm diameter x 5 cm high) to remove insoluble components. Elution with 2 x 10 mL of pentane, followed by evaporation under vacuum yielding pure products 2a-m.
References:
Romero, Erik A.;Jazzar, Rodolphe;Bertrand, Guy [Journal of Organometallic Chemistry,2017,vol. 829,p. 11 - 13] Location in patent:supporting information

767-91-9
120 suppliers
$10.00/250mg

73183-34-3
581 suppliers
$6.00/5g

149777-81-1
25 suppliers
inquiry