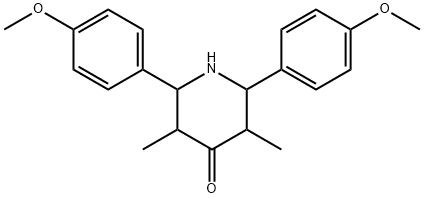
2,6-Bis(4-methoxyphenyl)-3,5-dimethylpiperidin-4-one synthesis
- Product Name:2,6-Bis(4-methoxyphenyl)-3,5-dimethylpiperidin-4-one
- CAS Number:151454-11-4
- Molecular formula:C21H25NO3
- Molecular Weight:339.43
Yield:151454-11-4 91%
Reaction Conditions:
with ammonium acetate in ethanol at 70; for 2 h;
Steps:
2.5. General procedure for the one-pot synthesis of 3,5-dialkyl-2,6-diarylpiperidin-4-ones 4
General procedure: A mixture of benzaldehyde (2 mmol), ammonium acetate(1 mmol), ketones (1 mmol) and the PS/TiCl4 containing certainamount of TiCl4 (0.15 gr, 0.1 mmol of TiCl4) in anhydrous ethanol(8 mL) was heated at 70 °C for an appropriate time as indicated by TLC (Table 2). After completion of the reaction, the mixture was cooled to room temperature and the resulting mixture was filtered to recover the catalyst and the filtrate was evaporated off the solvent to afford the crude product. The recovered catalyst was washed by ethanol, ether, and dried at 60 °C for 6 h and stored in a desiccator. The crude product obtained was washed with water and purified by recystallization from ethanol and ethanol-acetone (3:2,v/v) to afford the corresponding pure compound. 10 mmol-scale reactions were also carried out without any difficulties. All of the known products were characterized by comparison of their physical data, FTIR, and 1H NMR spectra with those of authentic samples.
References:
Rahmatpour, Ali;Emen, Reza;Amini, Ghazal [Journal of Organometallic Chemistry,2019,vol. 892,p. 24 - 33]
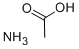
631-61-8
769 suppliers
$11.19/100G
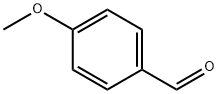
123-11-5
845 suppliers
$10.00/10g

96-22-0
274 suppliers
$9.00/1g

151454-11-4
6 suppliers
inquiry