
3',6'-Bis{[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)oxy]-3H-[spiro(isobenzofuran-1,9,-xanthen]-3-one} FBBBE synthesis
- Product Name:3',6'-Bis{[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)oxy]-3H-[spiro(isobenzofuran-1,9,-xanthen]-3-one} FBBBE
- CAS Number:1522117-83-4
- Molecular formula:C46H46B2O9
- Molecular Weight:764.47
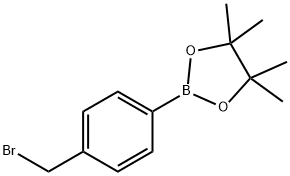
138500-85-3
255 suppliers
$8.00/1g
2321-07-5
355 suppliers
$6.00/25g
![3',6'-Bis{[4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)oxy]-3H-[spiro(isobenzofuran-1,9,-xanthen]-3-one} FBBBE](/CAS/20180808/GIF/1522117-83-4.gif)
1522117-83-4
10 suppliers
$28.00/1 mg
Yield:1522117-83-4 31%
Reaction Conditions:
with caesium carbonate
Steps:
General Methods
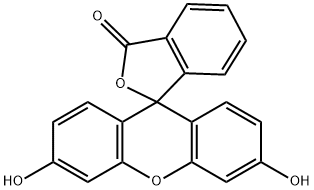
3',6'-bis((4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzyl)oxy)-3H-spiro[isobenzofuran-1,9'-xanthen]-3-one (FBBBE):
Fluorescein (2.45 g, 7.3 mmol) was dissolved in 50 mL MeCN.
To this was added Cs2CO3 (7.21 g, 22.1 mmol) followed by 4-bromomethylphenyl boronic acid pinacol ester (6.57 g, 22.1 mmol).
The reaction was held at reflux for 18 h under nitrogen.
The mixture was then cooled to room temperature, filtered, and rinsed with CH2Cl2.
The solvent was removed and the product was purified by silica gel chromatography, eluting with 20-30% EtOAc in hexanes to afford FBBBE in 31% yield (1.72 g, 2.3 mmol).
1H NMR (400 MHz, CDCl3): δ=8.01 (d, J=7.6 Hz, 1H), 7.85 (d, J=7.6 Hz, 4H), 7.62 (m, 2H), 7.43 (d, J=7.6 Hz, 4H), 7.15 (d, J=7.6 Hz, 1H), 6.82-6.81 (m, 2H), 6.70-6.67 (m, 4H), 5.11 (s, 4H), 1.35 (s, 24H).
13C NMR (100 MHz, CDCl3): δ=169.7, 160.5, 153.4, 152.6, 139.6, 135.3, 135.2, 129.9, 129.4, 127.0, 126.7, 125.2, 124.2, 112.5, 111.7, 102.1, 84.1, 83.4, 70.4, 25.1. ESI-MS (+): m/z 765.44 [M+H]+, 787.37 [M+Na]+.
References:
US2014/248218,2014,A1 Location in patent:Page/Page column