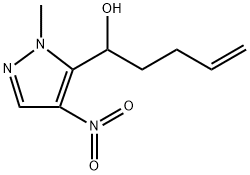
l-(l-methyl-4- nitro-lH-pyrazol-5-yl)pent-4-en-l-ol synthesis
- Product Name:l-(l-methyl-4- nitro-lH-pyrazol-5-yl)pent-4-en-l-ol
- CAS Number:1586044-15-6
- Molecular formula:C9H13N3O3
- Molecular Weight:211.22
Yield:1586044-15-6 36%
Reaction Conditions:
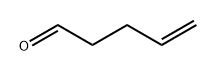
Stage #1: 4-pentenal;1-methyl-4-nitropyrazole in tetrahydrofuran at -78;Inert atmosphere;
Stage #2: with lithium hexamethyldisilazane in tetrahydrofuran at -78 - -40;
Steps:
Intermediate 68 l-(l-Methyl-4-nitro-lH-pyrazol-5-yl)pent-4-en-l-ol
A solution of l-methyl-4-nitro-lH-pyrazole (9.7 g, 76.7 mmol) and 4-pentenal (10 g, 84.4 mmol) in dry THF (250 mL) was cooled to -78 °C and stirred under nitrogen. A solution of LiHMDS (1 M in THF, 192 mL, 191.7 mmol) was added dropwise over a period of 3 hr. The reaction mixture was allowed to warm and to -40 °C and stirred for 2 hr, quenched by dropwise addition of saturated ammonium chloride solution (100 mL), warmed to room temperature and diluted with EtOAc (200 mL). The organic layer was washed with saturated ammonium chloride solution (50 mL), separated, dried over MgS04 and the solvent removed under reduced pressure. Purification via silica gel chromatography (0-100% EtOAc/DCM) followed by silica gel chromatography (0-100% EtOAc/isohexane) to gave l-(l-methyl-4- nitro-lH-pyrazol-5-yl)pent-4-en-l-ol as a pale yellow oil (5.75 g, 36%). NMR (400 MHz, CDC13) δ 8.06 (s, 1H), 5.85-5.78 (m, 1H), 5.32-5.26 (m, 1H), 5.12-5.04 (m, 2H), 3.98 (s, 3H), 3.45 (d, / = 8.7 Hz, 1H), 2.92-2.09 (m, 3H), 1.90-1.86 (m, 1H).
References:
WO2015/140189,2015,A1 Location in patent:Page/Page column 96-97