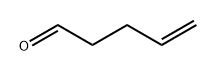
4-PENTENAL synthesis
- Product Name:4-PENTENAL
- CAS Number:2100-17-6
- Molecular formula:C5H8O
- Molecular Weight:84.12

79-37-8
463 suppliers
$17.67/10gm:

1972-28-7
378 suppliers
$15.00/5g

2100-17-6
86 suppliers
$56.20/1g
Yield:-
Reaction Conditions:
with triethylamine;triphenylphosphine;Al2O3 in tetrahydrofuran;dichloromethane;dimethyl sulfoxide
Steps:
6.g Example 6
g). The compound of formula XIII 5.4 g of the product of step f) above is added to 3.5 l of tetrahydrofuran at room temperature with stirring, 18.7 ml of diethyl azodicarboxylate is added followed by 31.3 g of triphenylphosphine and the mixture warmed to 50°C in an oil bath and allowed to react overnight. The product mixture is concentrated and the residue is purified using 2 Al2O3 columns and elution with hexane/ethyl acetate (2:1). Fractions 6-1 from the first column and fractions 7-20 from the second column together yield 5 g of crude product, which is further purified by silica gel chromatography to yield 3.2 g (62%) of the pure resinous intermediate product, being the product corresponding to the compound of formula XIII in which c is in the CH-OCH2-O-CH2-CH2-OCH3 protected form. This intermediate product is converted to the further intermediate product in which c is CHOH by treatment with acid. 5.2 g of the above intermediate product is dissolved in 12 ml of tetrahydrofuran, 10 ml of IN HCl is added and the mixture stirred at 45°C for 24 hr after which it is extracted three times with ethyl acetate and washed with saturated brine and NaHCO3 solutions. The ethyl acetate phases are dried over Na2SO4, concentrated and purified by silica gel chromatography (hexane/ethyl acetate 1:1) to give about 3.4 g of the sticky further intermediate product. 2 ml of dichloromethane is stirred at room temperature, 76 μl of oxalyl chloride is added, the mixture cooled to -60°C and a solution of 0.13 ml of DMSO in 1 ml of dichloromethane is added dropwise over 3 min. The resultant evolution of gas is allowed to proceed for 3 min after which a solution of 280 mg of the above further intermediate product in 3 ml of dichloromethane is added dropwise over 5 min with formation of a precipitate. The mixture is stirred for a further 15 min after which 0.56 ml of triethylamine is added over a 5 min period with the resultant precipitation of a paste which is allowed to accumulate at room temperature. The paste is extracted with dichloromethane, the organic phases washed twice with 10% citric acid twice with saturated brine solution, dried over Na2SO4, concentrated and purified by silica gel chromatography (dichloromethane) to give the title product of formula XIII in pure form. The pure product has the following characteristics: m. pt.159°C; m/z: 344(M+), 217(80), 189(60), 178(100), 95(60).
References:
SANDOZ LTD.;SANDOZ-PATENT-GMBH;SANDOZ-ERFINDUNGEN Verwaltungsgesellschaft m.b.H. EP606044, 1994, A1
821-09-0
248 suppliers
$21.21/1gm:

2100-17-6
86 suppliers
$56.20/1g

36842-44-1
3 suppliers
inquiry

2100-17-6
86 suppliers
$56.20/1g
39716-58-0
102 suppliers
$36.25/1g

2100-17-6
86 suppliers
$56.20/1g
201230-82-2
1 suppliers
inquiry
106-99-0
242 suppliers
$77.00/100mL

110-62-3
240 suppliers
$13.44/25ML

1072-21-5
54 suppliers
inquiry

1576-87-0
131 suppliers
$12.00/5g

2100-17-6
86 suppliers
$56.20/1g

7420-87-3
3 suppliers
inquiry

58838-14-5
3 suppliers
inquiry