
trans-3-Pentenal synthesis
- Product Name:trans-3-Pentenal
- CAS Number:58838-14-5
- Molecular formula:C5H8O
- Molecular Weight:84.12
201230-82-2
1 suppliers
inquiry

106-99-0
259 suppliers
$80.00/100mL

110-62-3
262 suppliers
$13.44/25ML

1072-21-5
55 suppliers
inquiry

1576-87-0
153 suppliers
$12.00/5g
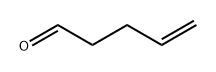
2100-17-6
106 suppliers
$95.12/1g

7420-87-3
3 suppliers
inquiry

58838-14-5
3 suppliers
inquiry
Yield:-
Reaction Conditions:
with (acetylacetonato)dicarbonylrhodium (l);4,5-bis(diphenylphosphinomethyl)-2,2-dimethyl-1,3-dioxolane;hydrogen in toluene at 80; under 15001.5 Torr; for 3 h;Autoclave;Reagent/catalyst;Temperature;
Steps:
2 Experimental
Hydroformylation reactions were conducted using a Parr Series 5000 Multiple Reactor System. 1,3-Butadiene was obtained as a solution (20wt.% in toluene) from Sigma-Aldrich. A typical hydroformylation of butadiene is performed in the following manner. Rh(acac)(CO)2 (4.77mg, 0.0184mmol), DIOP (46mg, 0.092mmol) and 5g of butadiene solution (20wt.% in toluene) were added to an autoclave in the glove box. The autoclave was sealed and removed from the glove box. The autoclave was flushed by syngas (CO/H2=1:1) at 5-6bar pressure. The procedure was repeated three times at the same pressure to ensure the complete replacement of the Ar by syngas, and then charged by the syngas to 20bar. The solution was stirred by 1000rpm while being heated at 80°C. The heating was continued for 3h. After 3h the autoclave was cooled to room temperature and the pressure was slowly released into a gas outlet valve inside a fumehood to avoid exposure to toxic CO. An aliquot was removed for immediate GC analysis and the product concentrations were determined from comparison to calibration curves. Pent-3-enal was produced by similar treatment of 15g of butadiene solution (20wt.% in toluene) with Rh(acac)(CO)2 (14.33mg, 0.056mmol) and diphenylphosphinoethane (dppe, 22.12mg, 0.056mmol) for 4h and purified by distillation at 150-160°C. The colorless solution obtained by this way was analyzed by GC and the concentration was determined from the calibration curve. The solution can be stored under argon at low temperature (-20°C) for 2-3days. A 5.5g solution of pent-3-enal in toluene (19.52mmol) was then treated with Rh(acac)(CO)2 (14.33mg, 0.056mmol), DIOP (55.17mg, 0.11mmol) at various temperatures and pressures outlined in Table 1. After 3h, an aliquot (150mg) was removed from the reaction mixture and added to a 10mL volumetric flask followed by 30mg of decane and diluted to 10mL with toluene. The sample was then analyzed by GC and the product concentrations were calculated from comparison to calibration curves. Gas chromatographic analysis was performed on Shimadzu QP2010 SE gas chromatograph equipped with a mass selective detector (GC-MS) using helium as carrier gas. A SHRXI-5MS (30m, 0.25mm ID, 0.25 um df) capillary column was used. The He flow rate was kept at 0.92mL/min. The column temperature was initially held at 60°C for 2min, then ramped at 10°C/min to 200°C and held at this temperature for 5min. Retention times (min) of the selected products are as follows: 1,3-butadiene (1.62), trans-pent-2-enal (2.71), trans-pent-3-enal (2.52), pent-4-enal (2.43), pentanal (2.55), 2-methylpentanedial (5.87), adipaldehyde (6.95).
References:
Maji, Tapan;Mendis, Camina H.;Thompson, Ward H.;Tunge, Jon A. [Journal of Molecular Catalysis A: Chemical,2016,vol. 424,p. 145 - 152]
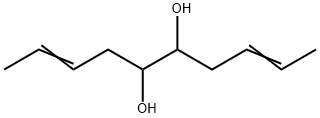
103723-04-2
0 suppliers
inquiry

58838-14-5
3 suppliers
inquiry
201230-82-2
1 suppliers
inquiry

106-99-0
259 suppliers
$80.00/100mL

110-62-3
262 suppliers
$13.44/25ML
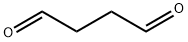
638-37-9
115 suppliers
inquiry

58838-14-5
3 suppliers
inquiry

764-37-4
37 suppliers
inquiry

58838-14-5
3 suppliers
inquiry