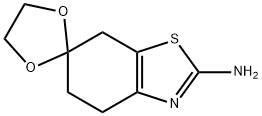
5,7-dihydro-4H-spiro[benzo[d]thiazole-6,2'-[1,3]dioxolan]-2-aMine synthesis
- Product Name:5,7-dihydro-4H-spiro[benzo[d]thiazole-6,2'-[1,3]dioxolan]-2-aMine
- CAS Number:159015-33-5
- Molecular formula:C9H12N2O2S
- Molecular Weight:212.27
Yield:159015-33-5 56%
Reaction Conditions:
with sulfur in methanol at 20;
Steps:
13
Stir a solution of cyanamide (2.80 g , 66.5 mmol) in 3 mL MeOH and add a solution of 1-(1,4- dioxa-spiro[4.5]dec-7-en-8-yl)-pyrrolidine (4.20 g, 66.5 mmol) and sulfur (2.13 g, 8.31 mmol) in 15 mL MeOH overnight at room temperature. Concentrate the mixture, dissolve in EtOAc and wash with H20. Extract the aqueous phase 2x EtOAc and wash with H20. Combine the organics and wash 3x aq. NH4C1 and lx H20. Re-extract aqueous phase with EtOAc, and wash with aq. NH4C1 and H20. Combine organics, dry with drying agent and concentrate to give a dark resin. Triturate with Et20 to afford 7.83 g, 56% yield of 2-amino-6-(l,4-dioxa-spiro)- 4,5,6,7-tetrahydrobenzothiazole as a solid. Add benzyl chloroformate (4.25 mL, 39.3 mmol) dropwise to a solution of 2-amino-6-(l,4- dioxa-spiro)-4,5,6,7-tetrahydrobenzothiazole (7.59 g, 35.8 mmol) and N,N- diisopropylethylamine (6.85 mL, 39.3 mmol) in 115 ml THF at 4 °C. Stir the mixture 0.5 h in the cold warming to room temperature under Ar overnight. Concentrate the mixture and dissolve the residue in EtOAc. Wash with lx H20 and 3x aq. NH4C1. Re-extract aqueous phase with 2x EtOAc. Wash the combined organics with aq. Na2C03, dry with a drying agent and concentrate to afford 11.0 g of a dark brown-resin. Purify by flash-chromatography eluting with CH2C12 to obtain 13.4 g of impure product. Triturate with acetone and filter to afford 8.90 g, 39% yield of (6-(l,4-dioxa-spiro)-4,5,6,7-tetrahydrobenzothiazol-2-yl)-carbamic acid benzyl ester as a solid.
References:
WO2012/54367,2012,A1 Location in patent:Page/Page column 62
![1,4-Dioxaspiro[4.5]decan-8-one](/CAS/GIF/4746-97-8.gif)
4746-97-8
468 suppliers
$5.00/1g
![5,7-dihydro-4H-spiro[benzo[d]thiazole-6,2'-[1,3]dioxolan]-2-aMine](/CAS/20150408/GIF/159015-33-5.gif)
159015-33-5
6 suppliers
inquiry
![Pyrrolidine, 1-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)-](/CAS/GIF/57440-57-0.gif)
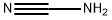
![(1,4-Dioxaspiro[4.5]dec-7-en-8-yloxy)-trimethylsilane](/CAS/GIF/144810-01-5.gif)

![Morpholine, 4-(1,4-dioxaspiro[4.5]dec-7-en-8-yl)-](/CAS/20180601/GIF/54621-20-4.gif)