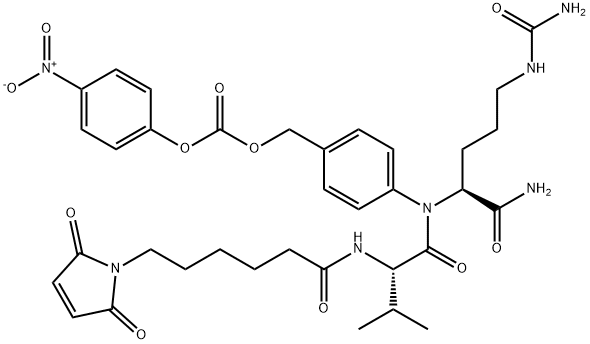
L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]- synthesis
- Product Name:L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]-
- CAS Number:159857-81-5
- Molecular formula:C35H43N7O11
- Molecular Weight:737.76
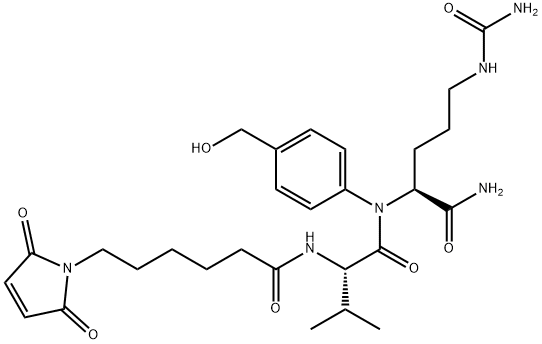
159857-80-4
174 suppliers
$27.00/1mg
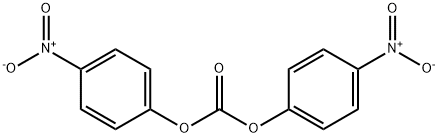
5070-13-3
232 suppliers
$5.00/5g
![L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]-](/CAS/20150408/GIF/159857-81-5.gif)
159857-81-5
195 suppliers
$11.00/1mg
Yield:159857-81-5 95%
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in N,N-dimethyl-formamide at 25;
Steps:
6 Example 6. Synthesis of mc-Val-Cit-PABC-PNP
mc-Val-Cit-PABOH (1 eq.) was mixed with bis(4-nitrophenyl) carbonate (1.87 eq.) in N,N-dimethylformamide (8 vol.) at 20°C. N,N-diisopropyl ethyl amine (1.75 eq.) was added at 25°C. The reaction mixture was stirred at 25°C for 2-6 hours until reaction was complete. Product was precipitated out of the reaction mixture by adding anhydrous ethyl acetate (12.5 vol.) at 25°C and tert-Butyl methyl ether (12.5 vol.). The resulting slurry was stirred, then cooled to 0°C and stirred for 10-30 minutes. The solids were isolated by filtration, washed and dried under vacuum before being re-slurried in ethyl acetate (12.5 vol.) at 20°C, filtered and dried once more (95% yield). MS: m/e 738 (MH)+, 760 (M+Na)+.
References:
SEATTLE GENETICS, INC.;BLANCHARD, Sophie;COATS, James WO2019/108797, 2019, A1 Location in patent:Paragraph 0117

623-04-1
349 suppliers
$6.00/1g
![L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]-](/CAS/20150408/GIF/159857-81-5.gif)
159857-81-5
195 suppliers
$11.00/1mg

55750-53-3
307 suppliers
$9.00/1g
![L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]-](/CAS/20150408/GIF/159857-81-5.gif)
159857-81-5
195 suppliers
$11.00/1mg
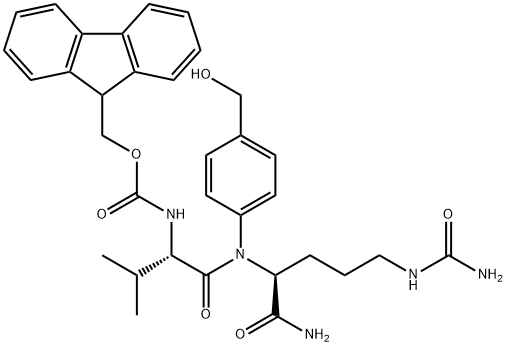
159858-22-7
197 suppliers
$14.00/25MG
![L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]-](/CAS/20150408/GIF/159857-81-5.gif)
159857-81-5
195 suppliers
$11.00/1mg
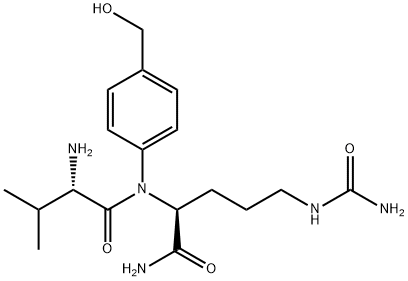
159857-79-1
183 suppliers
$32.00/5mg
![L-OrnithinaMide, N-[6-(2,5-dihydro-2,5-dioxo-1H-pyrrol-1-yl)-1-oxohexyl]-L-valyl-N5-(aMinocarbonyl)-N-[4-[[[(4-nitrophenoxy)carbonyl]oxy]Methyl]phenyl]-](/CAS/20150408/GIF/159857-81-5.gif)
159857-81-5
195 suppliers
$11.00/1mg