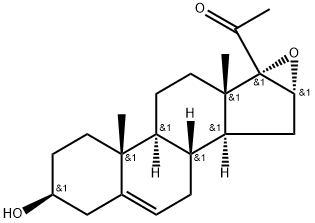
16,17-Epoxypregnenolone synthesis
- Product Name:16,17-Epoxypregnenolone
- CAS Number:974-23-2
- Molecular formula:C21H30O3
- Molecular Weight:330.47
979-02-2
285 suppliers
$20.00/100mg

974-23-2
101 suppliers
$116.00/10g
Yield: 97%
Reaction Conditions:
with sodium hydroxide;urea hydrogen peroxide adduct in methanol;water at 5; for 72 h;
Steps:
1
16-Dehydropregnenolone acetate (35 g) is suspended in methanol (500 ml). The solution is treated, after cooling to 500° C. with 4N NaOH (8.9 gm in 50 ml H2O) followed by immediately with hydrogen peroxide-urea adduct (UHP, 18 g.). The mixture is then stored in the refrigerator at 5° C. for 72 hr. The reaction mixture is shaken intermittently. The reaction mixture is poured into 500 ml of ice water. The product is isolated by centrifugation after wash up with water till neutrality to pH paper. The product is dried (63.0 g, 97%) M.P. 187-90 (187-90°). [0029] 1H-NMR (CDCl3): δ 5.3 (m, 1H, olefinic proton), 3.67 (s, 1H, C16-H), 3.5 (m, 1H, C3-H), 2.0 (s, 3H, CH3CO); 1.2 and 1.0 (2s, 3H each, C18Me, C19Me).
References:
COUNCIL OF SCIENTIFIC AND INDUSTRIAL RESEARCH US2004/186306, 2004, A1 Location in patent:Page 4
1162-53-4
126 suppliers
$57.00/1ea

974-23-2
101 suppliers
$116.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

974-23-2
101 suppliers
$116.00/10g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

974-23-2
101 suppliers
$116.00/10g
