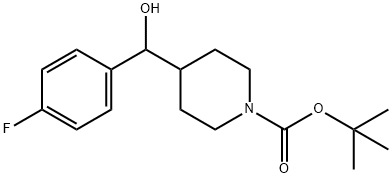
Tert-Butyl 4-((4-fluorophenyl)(hydroxy)methyl)piperidine-1-carboxylate synthesis
- Product Name:Tert-Butyl 4-((4-fluorophenyl)(hydroxy)methyl)piperidine-1-carboxylate
- CAS Number:160296-41-3
- Molecular formula:C17H24FNO3
- Molecular Weight:309.38
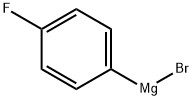
352-13-6
197 suppliers
$40.00/50 mL
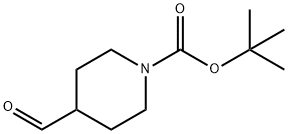
137076-22-3
367 suppliers
$5.00/1g

160296-41-3
24 suppliers
$250.00/1g
Yield: 82%
Reaction Conditions:
in diethyl ether at 0 - 20; for 1 h;Inert atmosphere;
Steps:
x92 x92: ferf-biitvl 4-ii4-fluorophenvl)ihydroxv)methvl)piperidine-
[0248] A solution of (4-fluorophenyl)magnesium bromide (23.44 mL, 18.76 mmol) in ether (46.9 mL) at 0°C under N2 atmosphere was treated with a solution of tert-hxityl 4- fomiylpiperidine-l-carboxylate (2 g, 9.38 mmol) in ether (4 mL) dropwise. Once the addition was complete the reaction mixture was allowed to warm up to RT and was stirred for 1 hour. The reaction mixture was quenched with saturated (aq) NH4CI and extracted with ether (1 x 150 mL) and EtOAc (1 x 2,00 mL). The organic layers were combined, dried over MgS04, filtered and the solvent removed under reduced pressure. The product was purified by column chromatography (dry packing) eluting with a gradient of 0-100% EtOAc in heptanes to give the title compound as a light yellow oil (2.37 g, 82%). ESI-MS m/z [M+H]+ 310.2.
References:
TAKEDA PHARMACEUTICAL COMPANY LIMITED;GREEN, Jason;HOPKINS, Maria;JONES, Benjamin;KIRYANOV, Andre A.;KUEHLER, Jon;MONENSCHEIN, Holger;MURPHY, Sean;NIXEY, Thomas;SUN, Huikai WO2018/183145, 2018, A1 Location in patent:Paragraph 0248

160296-40-2
80 suppliers
$45.00/50mg

160296-41-3
24 suppliers
$250.00/1g
56346-57-7
92 suppliers
$25.00/5g

160296-41-3
24 suppliers
$250.00/1g
![1-[4-(2,4-DIFLUORO-BENZOYL)-PIPERIDIN-1-YL]-ETHANONE](/CAS/GIF/25519-77-1.gif)
25519-77-1
19 suppliers
$446.00/25g

160296-41-3
24 suppliers
$250.00/1g
59084-16-1
115 suppliers
$14.14/250mgs:

160296-41-3
24 suppliers
$250.00/1g