
5,7-DICHLORO-4-HYDROXY-QUINOLINE-2-CARBOXYLIC ACID ETHYL ESTER synthesis
- Product Name:5,7-DICHLORO-4-HYDROXY-QUINOLINE-2-CARBOXYLIC ACID ETHYL ESTER
- CAS Number:166981-79-9
- Molecular formula:C12H9Cl2NO3
- Molecular Weight:286.11
![2-Butenedioic acid, 2-[(3,5-dichlorophenyl)amino]-, 1,4-diethyl ester, (2Z)-](/CAS/20210305/GIF/166753-63-5.gif)
166753-63-5
0 suppliers
inquiry

166981-79-9
3 suppliers
inquiry
Yield:166981-79-9 85.8%
Reaction Conditions:
with diphenylether at 244; for 0.166667 h;
Steps:
2
A 5-L three-neck flask equipped with a thermocouple, an addition runnel, and a distillation apparatus was charged with diphenyl ether (about 2.8 L), which was heated to about 244 °C. The concentrated reaction mixture containing Compound 3 (about 1.1 mole) from Reaction 1, above, was added from the dropping funnel into the hot diphenyl ether over about 10 minutes with a nitrogen purge. The funnel was rinsed with about 200 mL of diphenyl ether, which was also added to the flask. The resulting mixture was heated at about 244 0C for about 1 hour to cyclize Compound 3 to form Compound 4. The reaction mixture then was cooled to room temperature, at which point Compound 4 crystallized out. The crystallized product was isolated and slurried with ethyl acetate (EtOAc; about 1 L). The crystals were collected and rinsed with EtOAc (3 x 500 mL), and then dried under high vacuum at room temperature to afford about 269.95 g of Compound 4 (85.8 % yield over two steps).
References:
WO2007/44682,2007,A2 Location in patent:Page/Page column 9
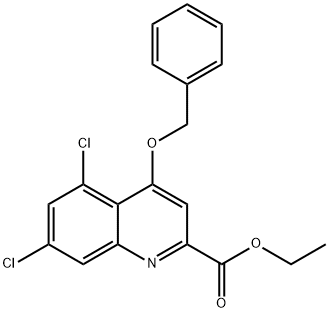
166981-77-7
0 suppliers
inquiry

166981-79-9
3 suppliers
inquiry
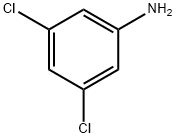
626-43-7
317 suppliers
$10.00/1g

166981-79-9
3 suppliers
inquiry

762-21-0
240 suppliers
$24.00/5G

166981-79-9
3 suppliers
inquiry