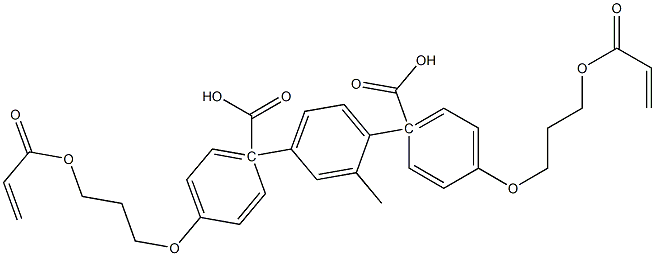
1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene synthesis
- Product Name:1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene
- CAS Number:174063-87-7
- Molecular formula:C33H32O10
- Molecular Weight:588.6
245349-46-6
53 suppliers
inquiry
95-71-6
398 suppliers
$9.00/25g
![1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene](/CAS/GIF/174063-87-7.gif)
174063-87-7
166 suppliers
$19.00/100mg
Yield:-
Reaction Conditions:
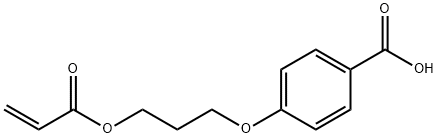
Stage #1: 4-(3-acryloyloxypropyloxy)benzoic acidwith methanesulfonyl chloride;N-ethyl-N,N-diisopropylamine in tetrahydrofuran;ethyl acetate; for 1 h;Cooling with ice;
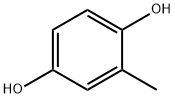
Stage #2: 2-methylbenzene-1,4-diolwith 1-methyl-1H-imidazole;triethylamine in tetrahydrofuran;ethyl acetate at 20; for 2 h;Cooling with ice;
Steps:
94 Synthesis of Liquid Crystal Composition
General procedure: Synthesis of Liquid Crystal Composition Compound (IV-1) (54 g, 204 mmol) and Compound (V-29) (6.8 g, 17.7 mmol) were mixed with ethyl acetate (50 mL), THF (45 mL) and diisopropylethylamine (41.8 mL). The obtained solution was added dropwise to an ethyl acetate solution of methanesulfonyl chloride (25.5 g, 223 mmol), slowly under cooling on ice. The feed ratio by mole of Compound (IV-1) and Compound (V-1) was 92:8. Next, the mixture was stirred for one hour under cooling on ice, an ethyl acetate solution of Compound (III-1) (13.5 g, 109 mmol) was added dropwise under cooling on ice, N-methylimidazole (0.5 g) was further added, and thereto triethylamine (33.7 mL) was slowly added dropwise under cooling on ice. The mixture was then stirred for two hours while keeping the reaction temperature at 20° C., water (140 mL) was added for extraction into an organic layer, and the organic layer was washed with a 2% aqueous hydrochloric acid solution and a 10% brine in this order. A portion of the organic layer was sampled and subjected to HPLC analysis, and production ratio of Compound (I-1) and Compound (II-53) was estimated based on the ratio of peak areas. The production ratio by mole was found to be 88:12. The obtained result was listed in Table 2 below. Next, the organic layer was filtered under suction, methanol/water was added to the filtrate so as to allow crystal to deposit, and the resultant crystal was collected by filtration, to thereby obtain a liquid crystal composition containing Compound (I-1) and Compound (II-53) (yield=60 g). The obtained liquid crystal composition was sampled and subjected to HPLC analysis, and compositional ratio of Compound (I-1) and Compound (II-53) was estimated based on the ratio of peak areas. The compositional ratio by mass was found to be 87:113. The obtained result was listed in Table 2 below. The liquid crystal composition was found to show a nematic-Iso phase transition temperature of 140° C. Note that the production ratio and the compositional ratio of Compound (I-1) and Compound (II-53) were estimated using standard curves determined based on the ratio of peak areas in HPLC analyses, using standard samples of separately synthesized Compound (I-1) and Compound (II-53).
References:
US9464228,2016,B2 Location in patent:Page/Page column 49-52

245349-46-6
53 suppliers
inquiry
![1,4-Bis-[4-(3-acryloyloxypropyloxy)benzoyloxy]-2-methylbenzene](/CAS/GIF/174063-87-7.gif)
174063-87-7
166 suppliers
$19.00/100mg