4-(3-ACRYLOYLOXY-N-PROP-1-YLOXY)BENZOIC ACID synthesis
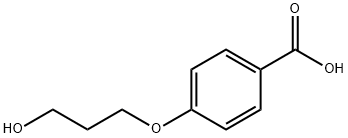
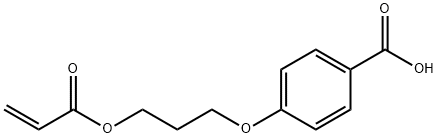
- Product Name:4-(3-ACRYLOYLOXY-N-PROP-1-YLOXY)BENZOIC ACID
- CAS Number:245349-46-6
- Molecular formula:C13H14O5
- Molecular Weight:250.25

Yield:245349-46-6 94 g
Reaction Conditions:
with methanesulfonic acid;2,6-di-tert-butyl-4-methyl-phenol in hexane at 70 - 80;
Steps:
1 Synthesis of intermediate 2
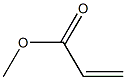
Insert stirrer, a reflux condenser, distillation, fractional distillation tube, the intermediate 1 In a reaction vessel equipped with a thermometer 98g (500mmol), methyl acrylate, 1290g, hexane 75g, 70% methanesulfonic acid 14g, butylhydroxytoluene 1.2g, It was reacted under stirring at 70 ~ 80 . The reaction is extracted with methanol produced by the reaction from the top of the distillation column to the fractional distillation tube, it was done until there is no production of methanol. The reaction solution was subjected to washing until the pH of water in 200ml of water was transferred to a 3 after cooling to room temperature, the separating funnel. The organic phase was concentrated at 65 ~ 75 under atmospheric pressure was added to 100g of toluene to obtain a slurry liquid. After the slurry liquid is separated by filtration, toluene was then washed with hexane and dried under reduced pressure to give 94g of Intermediate 2.
References:
KR2015/13217,2015,A Location in patent:Paragraph 0107-0109
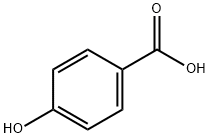
99-96-7
765 suppliers
$5.00/10g

245349-46-6
52 suppliers
inquiry
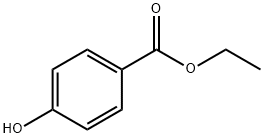
120-47-8
669 suppliers
$5.00/100mg

245349-46-6
52 suppliers
inquiry