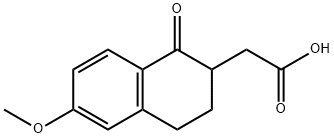
6-METHOXY-1-OXO-1,2,3,4-TETRAHYDRONAPHTHALEN-2-YL)ACETIC ACID synthesis
- Product Name:6-METHOXY-1-OXO-1,2,3,4-TETRAHYDRONAPHTHALEN-2-YL)ACETIC ACID
- CAS Number:17529-16-7
- Molecular formula:C13H14O4
- Molecular Weight:234.25
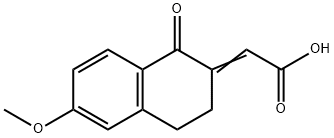
13587-70-7
3 suppliers
inquiry

17529-16-7
10 suppliers
inquiry
Yield:17529-16-7 95%
Reaction Conditions:
with acetic acid;zinc in water at 80; for 2 h;
Steps:
79
10560] Zinc (19.6 g, 300 mmol) was added to a solution of product of Example 79A (28 g, 120 mmol) in acetic acid- water mixture (224 mE+84 mE), and the mixture was stirred at 80° C. for 2 h. The reaction mixture was then filtered over celite bed, and the organic layer was removed under reduced pressure. Water (50 mE) was added to the residue. The resulting solids were collected by filtration and dried under vacuum to afford title compound (27 g, 95%) as solid. ‘H NMR (400 MHz, DMSO-d5): ? 12.2 (bs, 1H), 7.8 (d, J=8.4 Hz, 1H), 6.9 (m,2H),3.8(s,3H),3.1 (m, 1H),3.0-2.8 (m, 2H), 2.7 (m, 1H), 2.4 (m, 1H), 2.2 (m, 1H), 1.9 (m, 1H).
References:
US2015/307445,2015,A1 Location in patent:Paragraph 0555; 0559; 0560
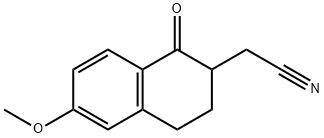
72605-00-6
0 suppliers
inquiry

17529-16-7
10 suppliers
inquiry
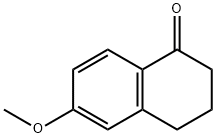
1078-19-9
364 suppliers
$6.00/5g

17529-16-7
10 suppliers
inquiry
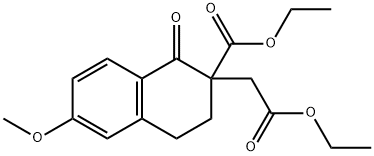
57350-98-8
0 suppliers
inquiry

17529-16-7
10 suppliers
inquiry
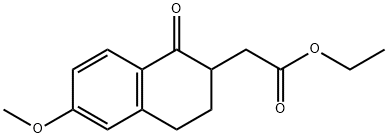
50558-96-8
3 suppliers
inquiry

17529-16-7
10 suppliers
inquiry