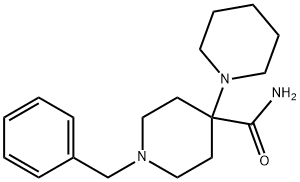
1'-(phenylmethyl)-[1,4'-bipiperidine]-4'-carboxamide synthesis
- Product Name:1'-(phenylmethyl)-[1,4'-bipiperidine]-4'-carboxamide
- CAS Number:1762-50-1
- Molecular formula:C18H27N3O
- Molecular Weight:301.43
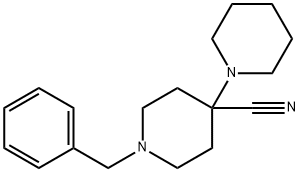
84254-97-7
35 suppliers
$50.00/25mg
![1'-(phenylmethyl)-[1,4'-bipiperidine]-4'-carboxamide](/CAS/GIF/1762-50-1.gif)
1762-50-1
14 suppliers
$149.90/10mg
Yield:1762-50-1 92%
Reaction Conditions:
Stage #1: 1'-benzyl-octahydro-[1,4']bipyridinyl-4'-carbonitrilewith sulfuric acid in water at 100; for 0.166667 h;Heating / reflux;
Stage #2: with sodium hydroxide in water at 0; pH=9;
Steps:
13
4.5 g (52.8 mmole) of piperidine, 5 g (26.4 mmole) N-benzyl-piperi-din-4-one, 9.5 g (3 eq) of magnesium sulfate and 2.3 ml of N-dimethylacetamide were mixed together and then 2.6 ml (1 eq) of 2-cyano-2-hydroxy-propane were added. The resulting suspension was stirred over 48 h at 55° C. whereupon pasty suspension solidifies. Crude product was mixed with 100 ml water and 100 ml ethyl acetate. Organic phase washed with water (2×50 ml), dried over sodium sulfate and evaporated yielding 7 g crude aminonitrile. 1.3 g (3.6 mmole) of the resulting aminonitrile was dissolved in 15 ml 90% wt. sulfuric acid and heated for 10 minutes at 100° C. The resulting solution was poured on ice and then basicified to pH 9 with sodium hydroxide. Crude amine was extracted with ethyl acetate (3×30 ml), organic phase washed once with a saturated solution of sodium chloride in water, dried over sodium sulfate and evaporated. Yield 1 g (92%) light yellow crystals of 1'-benzyl-1,4'-bipiperidine-4'-carboxamide.
References:
US2007/219214,2007,A1 Location in patent:Page/Page column 27

110-89-4
3 suppliers
$15.96/100ML
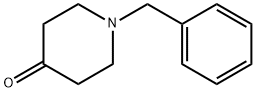
3612-20-2
489 suppliers
$6.00/10g
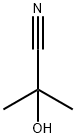
75-86-5
2 suppliers
$64.90/5G
![1'-(phenylmethyl)-[1,4'-bipiperidine]-4'-carboxamide](/CAS/GIF/1762-50-1.gif)
1762-50-1
14 suppliers
$149.90/10mg

3612-20-2
489 suppliers
$6.00/10g
![1'-(phenylmethyl)-[1,4'-bipiperidine]-4'-carboxamide](/CAS/GIF/1762-50-1.gif)
1762-50-1
14 suppliers
$149.90/10mg