
tert-butyl 4-[(2-ethoxy-2-oxoethyl)amino]tetrahydro-1(2H)-pyridinecarboxylate synthesis
- Product Name:tert-butyl 4-[(2-ethoxy-2-oxoethyl)amino]tetrahydro-1(2H)-pyridinecarboxylate
- CAS Number:177276-49-2
- Molecular formula:C14H26N2O4
- Molecular Weight:286.37
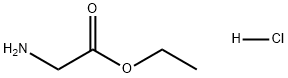
623-33-6
623 suppliers
$5.00/10g

79099-07-3
543 suppliers
$5.00/5g
![tert-butyl 4-[(2-ethoxy-2-oxoethyl)amino]tetrahydro-1(2H)-pyridinecarboxylate](/CAS/GIF/177276-49-2.gif)
177276-49-2
10 suppliers
$340.00/500mg
Yield:177276-49-2 77%
Reaction Conditions:
with methanol;sodium cyanoborohydride at 20; for 18 h;Inert atmosphere;
Steps:
[00188] Synthesis of tert-butyl 4-[(2-ethoxy-2-oxo-ethyl)amino]piperidine-1 - carboxylate
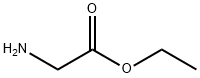
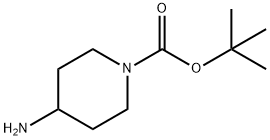
[00189] To a solution of tert-butyl 4-oxopiperidine-1-carboxylate (5.02 mmol, 1 eq) and glycine ethyl ester hydrochloride (5.02 mmol, 1 eq) in MeOH (2.5 ml) under nitrogen atmosphere was added sodium cyanoborohydride (6.02 mmol, 1.2 eq,) and the mixture at RT for 18 h. Then the reaction was quenched with ammonium chloride and concentrated in vacuo. The compound was extracted with dichloromethane, washed with sodium hydrogen carbonate, brine, dried over magnesium sulphate, filtered and concentrated in vacuo. The compound was purified by column chromatography eluting with dichloromethane with 0-5% methanol to give tert-butyl 4-[(2-ethoxy-2-oxo-ethyl)amino]piperidine-1-carboxylate (1.1 1 g, 77 %) as a colourless oil. AnalpH2_MeOH_4MIN: Rt: 1.64 min, m/z 287.3 [M+H]+
References:
WO2019/145718,2019,A1 Location in patent:Paragraph 00188-00189

79099-07-3
543 suppliers
$5.00/5g
459-73-4
50 suppliers
inquiry
![tert-butyl 4-[(2-ethoxy-2-oxoethyl)amino]tetrahydro-1(2H)-pyridinecarboxylate](/CAS/GIF/177276-49-2.gif)
177276-49-2
10 suppliers
$340.00/500mg
87120-72-7
20 suppliers
$6.00/1g

105-36-2
343 suppliers
$5.00/5G
![tert-butyl 4-[(2-ethoxy-2-oxoethyl)amino]tetrahydro-1(2H)-pyridinecarboxylate](/CAS/GIF/177276-49-2.gif)
177276-49-2
10 suppliers
$340.00/500mg