Spiro[1,3-dioxolane-2,2'-[2H]indene], 1',3'-dihydro- synthesis
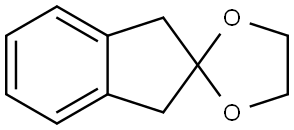
- Product Name:Spiro[1,3-dioxolane-2,2'-[2H]indene], 1',3'-dihydro-
- CAS Number:183-24-4
- Molecular formula:C11H12O2
- Molecular Weight:176.21
Yield:183-24-4 90%
Reaction Conditions:
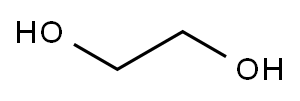
with toluene-4-sulfonic acid;orthoformic acid triethyl ester at 20; for 3 h;
Steps:
7.2.1. 1',3'-Dihydro-spiro[[1,3]dioxolane-2,2'-indene] (4)
To a solution of 2-indanone (2 g, 15.2 mmol) in ethylene glycol (10 mL) was added triethylorthoformate (5 mL) and a crystal of TsOH. The reaction was stirred at room temperature for 3 h, and the solvent removed in vacuo. The residue was purified by flash column chromatography on silica gel (eluant: pet ether:ethyl acetate, 5:1) to yield the dioxane, (2.4 g, 90%), a colourless oil with the following physical properties: IR (film) νmax/cm-1 2955, 2885, 1403, 1331, 1292, 1226, 1104, 1030 cm-1; 1H NMR (CDCl3, 400 Hz) δH 3.27 (4H, s, 2× CH2), 4.06 (4H, s, 2× OCH2), 7.23-7.28 (4H, m, 4× Ar-CH); 13C NMR δC 42.84 (2× CH2), 64.07 (2× OCH2), 117.11 (OCO), 124.14 (2× Ar-CH), 126.25 (2× Ar-CH), 139.45 (2× Ar-C); MS, m/z, (RI) 177 (M+1, 28), 176 (M+, 100), 117 (12), 104 (42).
References:
Barlow, James W.;McHugh, Aengus P.;Woods, Orla;Walsh, John J. [European Journal of Medicinal Chemistry,2011,vol. 46,# 5,p. 1545 - 1554] Location in patent:experimental part