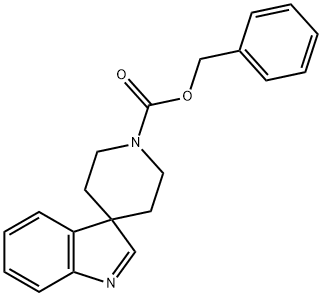
benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate synthesis
- Product Name:benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate
- CAS Number:184289-85-8
- Molecular formula:C20H20N2O2
- Molecular Weight:320.39
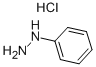
59-88-1
356 suppliers
$15.00/10g
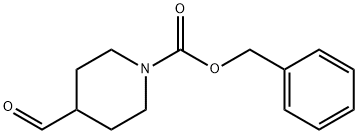
138163-08-3
187 suppliers
$6.00/1g
![benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/184289-85-8.gif)
184289-85-8
10 suppliers
inquiry
Yield:184289-85-8 54%
Reaction Conditions:
with trifluoroacetic acid in dichloromethane at 0 - 20; for 17 h;
Steps:
Synthesis of spiroindolenines 3
General procedure: To an ice-cooled solution of carbaldehyde (1 eq.) in DCM (0.1 M), the corresponding aryl hydrazinehydrochloride (1 eq.) was slowly added. After 10 min at 0°C, TFA (2 eq) was added dropwise. Thereaction mixture was stirred at room temperature overnight. The mixture was diluted with DCM andwater and Na2CO3 (3 eq.) were added. The organic phase was separated and washed with water andbrine. Finally, it was dried over Na2SO4 and concentrated under reduced pressure. The crude productwas subjected to column chromatography (SiO2, corresponding eluent).
References:
Estévez, Verónica;Kloeters, Laura;Kwietniewska, Natalia;Vicente-García, Esther;Ruijter, Eelco;Orru, Romano V.A. [Synlett,2017,vol. 28,# 3,art. no. ST-2016-D0595-L,p. 376 - 380]

138163-08-3
187 suppliers
$6.00/1g
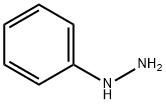
100-63-0
358 suppliers
$10.00/1g
![benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/184289-85-8.gif)
184289-85-8
10 suppliers
inquiry
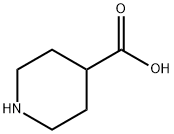
498-94-2
1 suppliers
$5.00/10g
![benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/184289-85-8.gif)
184289-85-8
10 suppliers
inquiry
![1-[(Benzyloxy)carbonyl]piperidine-4-carboxylic acid](/CAS/GIF/10314-98-4.gif)
10314-98-4
262 suppliers
$6.00/1g
![benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/184289-85-8.gif)
184289-85-8
10 suppliers
inquiry

10314-99-5
68 suppliers
$65.25/250mg
![benzyl spiro[indole-3,4'-piperidine]-1'-carboxylate](/CAS/20150408/GIF/184289-85-8.gif)
184289-85-8
10 suppliers
inquiry