
6-AMINO-4-(4-METHOXYPHENYL)-1,3-DIPHENYL-1,4-DIHYDROPYRANO[2,3-C]PYRAZOLE-5-CARBONITRILE synthesis
- Product Name:6-AMINO-4-(4-METHOXYPHENYL)-1,3-DIPHENYL-1,4-DIHYDROPYRANO[2,3-C]PYRAZOLE-5-CARBONITRILE
- CAS Number:188199-07-7
- Molecular formula:C26H20N4O2
- Molecular Weight:420.46
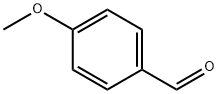
123-11-5
861 suppliers
$10.00/10g
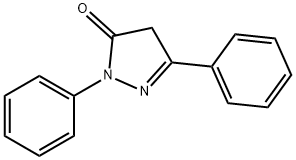
4845-49-2
36 suppliers
inquiry

109-77-3
429 suppliers
$10.00/5g
![6-AMINO-4-(4-METHOXYPHENYL)-1,3-DIPHENYL-1,4-DIHYDROPYRANO[2,3-C]PYRAZOLE-5-CARBONITRILE](/StructureFile/ChemBookStructure4/GIF/CB1711525.gif)
188199-07-7
6 suppliers
inquiry
Yield:188199-07-7 87%
Reaction Conditions:
Stage #1: 4-methoxy-benzaldehyde;malononitrilewith triethylamine in ethanol; for 0.0166667 h;
Stage #2: 1,3-diphenyl-5-oxo-4,5-dihydro-1H-pyrazole in ethanol; for 24 h;
Steps:
6-amino-4-(4-methoxyphenyl)- 1-methyl-3-phenyl- 1 ,4-dihydropyrano [2,3-cj pyrazole25 5-carbonitrile UC-E28
6-amino-4-(4-methoxyphenyl)- 1-methyl-3-phenyl- 1 ,4-dihydropyrano [2,3-cj pyrazole25 5-carbonitrile UC-E28: A mixture of anisaldehyde (350 iL, 2.87 mmol, 1.0 equ.),malononitrile (190 mg, 2.87 mmol, 1.0 equ.) and triethylamine (400 iL, 2.87 mmol, 1.0 equ.) in ethanol (10 mL) is stirred for 1 mm, followed by the addition of 1,3-diphenyl-1H- pyrazol-5(4H)-one (678 mg, 2.87 mmol, 1 equ.). The reaction mixture was concentrated after 24 h and the precipitate was washed with ethanol and hexanes. The product was re30 crystallized from ethanol to afford 6-amino-4-(4-methoxyphenyl)- 1 -methyl-3 -phenyl- 1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile (1.05 g, 87%, 2.50 mmol) as a white solid. ‘H-NMR (400 MHz) DMSO: 7.94-7.92 (d, 2H), 7.63-7.6 1 (d, 2H), 7.57-7.53 (t, 2H), 7.40-7.36 (t, 1H), 7.29-7.23 (m, 3H) 7.15 (s, 2H), 7.13-7.11 (d, 2H), 6.78-6.76 (d, 2H), 5.02 (s,1H), 3.65 (s, 3H), ‘3C-NMR (100 MHz) DMSO: 158.9, 158.4, 146.7, 145.6, 137.9, 136.5,132.6, 129.8, 129.0, 128.7, 128.5, 127.2, 127.0, 121.2, 120.3, 114.2, 98.3, 60.2, 55.3, 37.1;LC/MS-MS: 421.2 - 355.0 m/z; GS1 and GS2 at 30, DP = 81, CE = 35, CXP = 24, tR =4.32 mm.
References:
WO2013/96820,2013,A1 Location in patent:Paragraph 50; 51
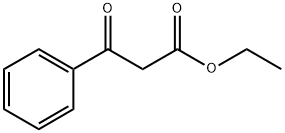
94-02-0
390 suppliers
$14.00/5g

123-11-5
861 suppliers
$10.00/10g
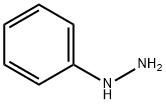
100-63-0
365 suppliers
$10.00/1g

109-77-3
429 suppliers
$10.00/5g
![6-AMINO-4-(4-METHOXYPHENYL)-1,3-DIPHENYL-1,4-DIHYDROPYRANO[2,3-C]PYRAZOLE-5-CARBONITRILE](/StructureFile/ChemBookStructure4/GIF/CB1711525.gif)
188199-07-7
6 suppliers
inquiry

100-63-0
365 suppliers
$10.00/1g
![6-AMINO-4-(4-METHOXYPHENYL)-1,3-DIPHENYL-1,4-DIHYDROPYRANO[2,3-C]PYRAZOLE-5-CARBONITRILE](/StructureFile/ChemBookStructure4/GIF/CB1711525.gif)
188199-07-7
6 suppliers
inquiry

94-02-0
390 suppliers
$14.00/5g
![6-AMINO-4-(4-METHOXYPHENYL)-1,3-DIPHENYL-1,4-DIHYDROPYRANO[2,3-C]PYRAZOLE-5-CARBONITRILE](/StructureFile/ChemBookStructure4/GIF/CB1711525.gif)
188199-07-7
6 suppliers
inquiry
