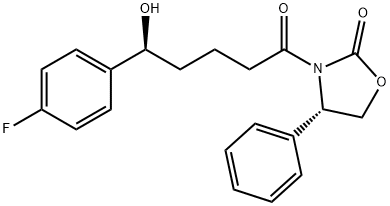
(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE synthesis
- Product Name:(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE
- CAS Number:189028-95-3
- Molecular formula:C20H20FNO4
- Molecular Weight:357.38
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
363 suppliers
$6.00/1g
![(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE](/CAS/GIF/189028-95-3.gif)
189028-95-3
280 suppliers
$18.00/1g
Yield:189028-95-3 97.23%
Reaction Conditions:
with D-glucose;lithium hydroxide monohydrate;butanoic acid ethyl ester at 30; pH=7; for 24 h;Temperature;Reagent/catalyst;
Steps:
1-14 Example 1
Add 151.20g (8400.00mmol) of water and 23.90g (132.66mmol) of glucose into a 500ml three-necked flask, and manually stir to dissolve.Add 90.00g (775.86mmol) ethyl butyrate and 30.00g (84.82mmol) (4S)-3-[5-(4-fluorophenyl)-1,5-dioxopentyl]-4-phenyl-2-oxazolidinone,Turn on mechanical stirring, set the speed to 500 rpm, set the temperature to 30°C (note: the reaction solution needs to be stirred into an emulsified state), and adjust the pH to 7.0 with a saturated sodium bicarbonate aqueous solution through a potentiometric titrator. The pH is stable at 7.0-7.5, 1.37g CpCR (enzyme) and 0.10g Easy/B (coenzyme) are added, and saturated aqueous sodium bicarbonate solution is added dropwise with a potentiometer to control the system to react at around pH 7.0. After 24 hours of reaction, adjust the pH to 2 with hydrochloric acid (1mol/L), add 200.0ml EA to extract the liquids, wash the organic phase with 200.0ml water, and concentrate the organic phase under reduced pressure to obtain 28.43g of oil, which is the target product (4S)-3-[(5S)-5-(4-fluorophenyl)-5-hydroxyvaleryl]-4-phenyl-1,3-oxazepine-2-one, yield It was 97.23%, and the HPLC purity was 99.22% (Figure 1). Calculated value C: 67.17%, H: 5.63%, O: 17.92%; detected value C: 67.20%, H: 5.58%, O: 17.99%.
References:
CN113072516,2021,A Location in patent:Paragraph 0021-0048

99395-88-7
496 suppliers
$5.00/5g

149437-76-3
367 suppliers
$8.00/10g
![(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE](/CAS/GIF/189028-95-3.gif)
189028-95-3
280 suppliers
$18.00/1g
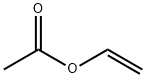
108-05-4
561 suppliers
$4.00/1000g
![2-Oxazolidinone, 3-[5-(4-fluorophenyl)-5-hydroxy-1-oxopentyl]-4-phenyl-, (4S)-](/CAS/GIF/1246853-48-4.gif)
1246853-48-4
0 suppliers
inquiry
![2-Oxazolidinone, 3-[(5R)-5-(acetyloxy)-5-(4-fluorophenyl)-1-oxopentyl]-4-phenyl-, (4S)-](/CAS/20200611/GIF/1246853-49-5.gif)
1246853-49-5
0 suppliers
inquiry
![(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE](/CAS/GIF/189028-95-3.gif)
189028-95-3
280 suppliers
$18.00/1g
67-63-0
1552 suppliers
$17.00/25ML
![(4S)-3-[5-(4-Fluorophenyl)-1,5-dioxopenyl]-4-phenyl-2-oxazolidinone](/CAS/GIF/189028-93-1.gif)
189028-93-1
363 suppliers
$6.00/1g
![(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE](/CAS/GIF/189028-95-3.gif)
189028-95-3
280 suppliers
$18.00/1g

149437-76-3
367 suppliers
$8.00/10g
![(4S)-3-[(5R)-5-(4-FLUOROPHENYL)-5-HYDROXYPENTANOYL]-4-PHENYL-1,3-OXAZOLIDIN-2-ONE](/CAS/GIF/189028-95-3.gif)
189028-95-3
280 suppliers
$18.00/1g