
4-BENZYLIDENE-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER synthesis
- Product Name:4-BENZYLIDENE-PIPERIDINE-1-CARBOXYLIC ACID TERT-BUTYL ESTER
- CAS Number:193217-39-9
- Molecular formula:C17H23NO3
- Molecular Weight:289.37

37586-22-4
72 suppliers
$541.82/5g

24424-99-5
850 suppliers
$13.50/25G

193217-39-9
72 suppliers
$75.00/100mg
Yield: 90%
Reaction Conditions:
Stage #1:4-benzoylpiperidine with sodium hydrogencarbonate in water at 20; for 0.25 h;
Stage #2:di-tert-butyl dicarbonate in water at 20; for 15 h;
Steps:
6
To a mixture of NaHC03 (173 g) and water (1.5 L) was added compound 11a of J. Med. Chem. 2003, vol 46, 25, 5512-5532 (119.3 g, 0.63 mol). The reaction was stirred at room temperature for 15 min and then (Boc)20 was added. After stirring for 15 h at room temperature a white solid was filtered and washed with water to affor 164 g (Yield: 90 %, mp 91-93 °C) of 4-benzoyl-l-[(l,l-dimethylethyloxy)carbonyl]piperidine. A suspension of Mg turnings (1.0 g) in anhydrous diethyl ether (40 mL) was prepared and treated with 1/3 of a solution of 4-chlorobenzyl chloride (4.6 g, 27.0 mmol) in anhydrous diethyl ether (40 mL) and an iodine crystal. The mixture was heated until a smooth reflux was observed and the color disappeared. The rest of the solution of 4- chlorobenzyl chloride was added. The reflux was continued for 3.5 h and the reaction mixture cooled to room temperature. A solution of 4-benzoyl-l-[(l,l- dimethylethyloxy)carbonyl]piperidine (6.4 g, 22.4 mmol) in anhydrous diethyl ether (50 mL) was added dropwise and the reaction refluxed for 3h. A saturated aqueous NH4C1 solution (50 mL) was added, the diethyl ether evaporated and the mixture extracted with CHC13. The organic layer was dried over anhydrous Na2S04, filtered and concentrated in vacuo to give to give a solid which was washed with hexane. This solid was dissolved in chloroform (100 mL), treated with trifluoro acetic acid (8 mL) and refluxed for 15 h. The reaction mixture was cooled to room temperature, treated with 10 % aqueous NaOH solution and extracted with chloroform. The organic layer was dried over anhydrous Na2S04, filtered and evaporated under reduced pressure to afford a mixture E/Z isomers. Purification by flash chromatography on silica gel (Diethyl etherTsopropilamine 10/0.5) gave 1.6 g of the Z isomer of the title compound (Yield: 22 %, mp 121-124 °C).
References:
FAES FARMA, S.A.;LEDO GÓMEZ, Francisco;MUÑOZ MUÑOZ, Ana;PUMAR DURÁN, Carmen WO2011/147780, 2011, A1 Location in patent:Page/Page column 30

24424-99-5
850 suppliers
$13.50/25G
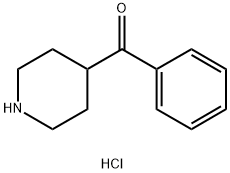
25519-80-6
89 suppliers
$22.32/Price $/250mgs:

193217-39-9
72 suppliers
$75.00/100mg
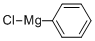
100-59-4
264 suppliers
$38.00/0.25mole
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
186 suppliers
$6.00/100mg

193217-39-9
72 suppliers
$75.00/100mg
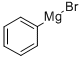
100-58-3
195 suppliers
$18.00/10ml
![1-Boc-4-[methoxy(methyl)carbamoyl]piperidine](/CAS/GIF/139290-70-3.gif)
139290-70-3
186 suppliers
$6.00/100mg

193217-39-9
72 suppliers
$75.00/100mg
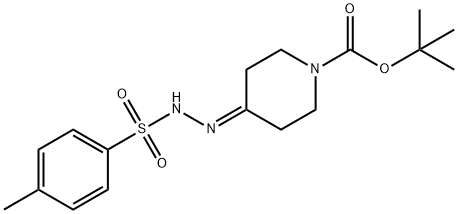
1046478-89-0
35 suppliers
$155.00/50mg

100-52-7
964 suppliers
$17.30/2g

193217-39-9
72 suppliers
$75.00/100mg