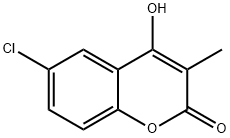
2H-1-Benzopyran-2-one, 6-chloro-4-hydroxy-3-Methyl- synthesis
- Product Name:2H-1-Benzopyran-2-one, 6-chloro-4-hydroxy-3-Methyl-
- CAS Number:197504-51-1
- Molecular formula:C10H7ClO3
- Molecular Weight:210.61
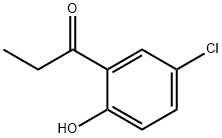
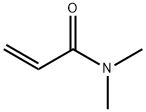
2892-16-2
47 suppliers
$110.00/50mg
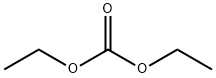
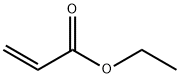
105-58-8
450 suppliers
$15.00/25g

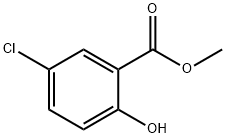
197504-51-1
6 suppliers
inquiry
Yield:197504-51-1 76%
Reaction Conditions:
Stage #1: 5'-chloro-2'-hydroxypropiophenone;Diethyl carbonatewith sodium hydride in toluene; for 20 h;Heating / reflux;
Stage #2: with sodium hydroxide;water in toluene at 20; for 60 h;
Stage #3: with hydrogenchloride in water; pH=2;
Steps:
39 6-Chloro-4-hydroxy-3-methyl-1-benzopyran-2-one
EXAMPLE 39 6-Chloro-4-hydroxy-3-methyl-1-benzopyran-2-one Diethylcarbonate (27.2 mmol, 3.3 mL) is dissolved in 30 mL toluene, and NaH (16.3 mmol, 0.65 g of 60% dispersion) is added in portions. 5'-chloro-2'-hydroxy-propiophenone (5.4 mmol, 1.0 g) in 10 mL is dissolved in toluene and is added dropwise via an addition funnel to the above solution. The cloudy yellow-green mixture is heated to near reflux for 20 hrs then is cooled to ambient temperature. 1N NaOH (50 mL) is added and the mixture stirred at ambient temperature for 60 hrs. The layers are separated and the organic layer is washed with H2O. The combined aqueous layers are washed with Et2O and the aqueous layer then acidified to pH 2 with conc. HCl. The resulting off-white precipitate is filtered, is washed with Et2O, and is dried to afford the title compound (0.417 g, 37%). A second crop is obtained by refrigerating the filtrate (0.442 g, 39%). 1H NMR (DMSO-d6, 300 MHz) δ 11.50 (1H, br s), 7.88 (1H, d, J=2.5 Hz), 7.61 (1H, dd, J=2.62, 8.87 Hz), 7.40 (1H, d, J=8.87 Hz), 2.00 (3H, s); MS (ESI, Pos.) calcd for C10H7ClO3 ml/z [M+H]=211.0, found 210.9.
References:
US2005/54681,2005,A1 Location in patent:Page/Page column 22