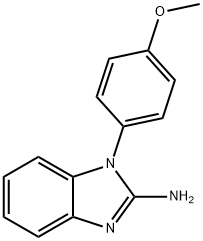
1H-Benzimidazol-2-amine, 1-(4-methoxyphenyl)- synthesis
- Product Name:1H-Benzimidazol-2-amine, 1-(4-methoxyphenyl)-
- CAS Number:33235-40-4
- Molecular formula:C14H13N3O
- Molecular Weight:239.27
Yield:33235-40-4 43%
Reaction Conditions:
with copper(l) iodide;8-quinolinol;caesium carbonate in tert-butyl alcohol at 90; for 16 h;Sealed tube;
Steps:
4.2.1 General procedure for the N-arylation of 2-aminobenzimidazole [21,26]
General procedure: To a 2-5mL microwave vial was added 2-aminobenzimidazole (150mg, 1.13mmol), Cs2CO3 (918mg, 2.82mmol, 2.5 eq.), CuI (21mg, 0.11mmol, 0.1 eq.), 8-hydroxyquinoline (25mg, 0.17mmol, 0.15 eq.) and the appropriately substituted bromo or iodobenzene (1.24mmol, 1.1 eq.) were suspended in t-BuOH (2mL). The vial was sealed and heated at 110°C (for bromobenzenes) or 90°C (for iodobenzenes) for 16h. The cooled mixture was poured into EtOAc/MeOH (20:1) (30mL), filtered through Celite and evaporated under reduced pressure. The residue was purified by column chromatography using DCM/MeOH/NH4OH (89:10:1) to give typically colourless amorphous solids. This method produced compounds 2a-2l, 3a-3r, 4a, 4b, 5a, 5b, 6a-6d, 7, 8a-8c, 10.
References:
Devine, Shane M.;Challis, Matthew P.;Kigotho, Jomo K.;Siddiqui, Ghizal;De Paoli, Amanda;MacRaild, Christopher A.;Avery, Vicky M.;Creek, Darren J.;Norton, Raymond S.;Scammells, Peter J. [European Journal of Medicinal Chemistry,2021,vol. 221,art. no. 113518]
615-36-1
416 suppliers
$10.00/5g

33235-40-4
3 suppliers
inquiry
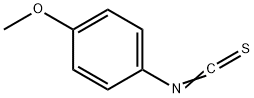
2284-20-0
137 suppliers
$12.00/1g

33235-40-4
3 suppliers
inquiry
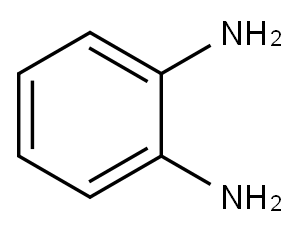
95-54-5
527 suppliers
$9.00/1g

33235-40-4
3 suppliers
inquiry