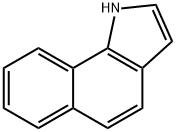
1H-BENZO(G)INDOLE synthesis
- Product Name:1H-BENZO(G)INDOLE
- CAS Number:233-34-1
- Molecular formula:C12H9N
- Molecular Weight:167.21
Yield:233-34-1 97%
Reaction Conditions:
with platinum on alumina;zinc oxide at 185; for 24 h;Sealed tube;regioselective reaction;
Steps:
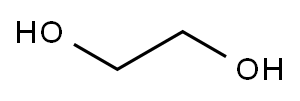
General Procedure A: Ethylene Glycol as Solvent
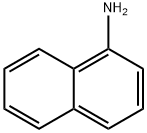
General procedure: The starting material (1-aminonaphthalene (1a), 2-aminonaphthalene (1b), 1-aminoanthracene (2a), or 2-aminoanthracene (2b)) (2 mmol), Pt/Al2O3 (132 mg, 1.7 mol%), ZnO dispersion (22 μL, 4.5 mol%), and ethylene glycol (5 mL) were mixed in a 50 mL glass reaction tube. The tube was sealed and put into a reaction station with stirring at 185 °C for 24 h. The reaction mixture was cooled to room temperature. A volume of 10 mL of ethyl acetate was added, and the crude mixture was filtered through a 0.2 m PTFE filter. The reaction mixture was extracted with brine (3 × 20 mL), and the organic layer was dried with Na2SO4, filtered, and concentrated under vacuum. The residue was purified, when required, by column chromatography on silica gel with hexane/ethyl acetate mixtures. The isolated compounds are described in order of elution.
References:
Gonzalez-Sanchis, Nerea;Perez-Quilez, Paula;Bellezza, Delia;Flor-Sanchez, Anna;Ballesteros, Rafael;Ballesteros-Garrido, Rafael [Synthesis,2022,vol. 54,# 23,p. 5226 - 5232]

176853-40-0
10 suppliers
inquiry

233-34-1
30 suppliers
$144.00/1g
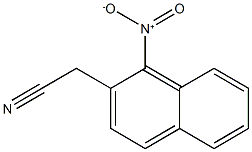
89278-00-2
5 suppliers
inquiry

233-34-1
30 suppliers
$144.00/1g

445262-76-0
0 suppliers
inquiry

233-34-1
30 suppliers
$144.00/1g