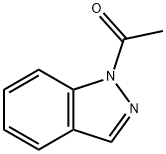
1H-Indazole, 1-acetyl- (7CI,8CI,9CI) synthesis
- Product Name:1H-Indazole, 1-acetyl- (7CI,8CI,9CI)
- CAS Number:13436-49-2
- Molecular formula:C9H8N2O
- Molecular Weight:160.17
18595-14-7
298 suppliers
$8.00/5g

108-24-7
5 suppliers
$14.00/250ML

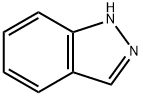
13436-49-2
35 suppliers
$118.00/1g
Yield:13436-49-2 1.2 g
Reaction Conditions:
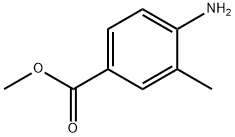
Stage #1: 4-methoxycarbonyl-2-methylaniline;acetic anhydride in chloroform at 0 - 40; for 1 h;
Stage #2: with anhydrous potassium acetate;isopentyl nitrite in dichloromethane at 70; for 18 h;
Steps:
3-1 Synthesis of 1H-indazol-5-carboxylic acid methyl ester
4-Amino-3-methyl-benzoic acid methyl ester (2.0 g, 12.03 mmol) was dissolved in chloroform (25 mL) and then acetic anhydride (2.12 g, 30.07 mmol) was slowly added dropwise thereto at 0°C. The mixture was stirred for 1 hour at room temperature, and potassium acetate (250 mg, 3.61 mmol) and isoamyl nitrite (2.23 mL, 24.06 mmol) were added thereto. The mixture was stirred under reflux for 18 hours at 70°C and added with excess dichloromethane. The mixture was washed with saturated sodium hydrogen carbonate aqueous solution, dried with anhydrous magnesium sulfate and filtered. The filtrate was distilled under reduced pressure. The residue was separated by column chromatography to obtain the intermediate acetyl indazole (1.2 g, 5.50 mmol).
References:
EP4050010,2022,A1 Location in patent:Paragraph 0057; 0074
13493-47-5
43 suppliers
$60.00/10mg

13436-49-2
35 suppliers
$118.00/1g
271-44-3
249 suppliers
$6.00/250mg

108-24-7
5 suppliers
$14.00/250ML

13436-49-2
35 suppliers
$118.00/1g

18595-14-7
298 suppliers
$8.00/5g
110-46-3
288 suppliers
$10.00/10g

13436-49-2
35 suppliers
$118.00/1g

108-24-7
5 suppliers
$14.00/250ML
95-53-4
437 suppliers
$10.00/1g

13436-49-2
35 suppliers
$118.00/1g