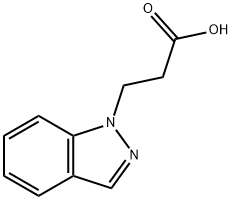
1H-Indazole-1-propanoic acid synthesis
- Product Name:1H-Indazole-1-propanoic acid
- CAS Number:247128-24-1
- Molecular formula:C10H10N2O2
- Molecular Weight:190.2
![1H-Pyrrole-2,5-dione, 3-[1-(3-hydroxypropyl)-1H-indazol-3-yl]-4-[1-(3-pyridinyl)-1H-indol-3-yl]-](/CAS/20210305/GIF/436161-39-6.gif)
436161-39-6
0 suppliers
inquiry
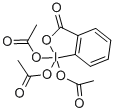
87413-09-0
516 suppliers
$10.64/1G

247128-24-1
9 suppliers
$378.00/1g
Yield:247128-24-1 98%
Reaction Conditions:
with sodium thiosulfate in dichloromethane;water;
Steps:
34 1H-indazole-1-propanoic acid, 3-[2,5-dihydro-2,5-dioxo-4-[1-(3-pyridinyl)-1H-indol-3-yl]-1H-pyrrol-3-yl]-1H-pyrrole-2,5-dione (Compound 113)
1H-indazole-1-propanoic acid, 3-[2,5-dihydro-2,5-dioxo-4-[1-(3-pyridinyl)-1H-indol-3-yl]-1H-pyrrol-3-yl]-1H-pyrrole-2,5-dione (Compound 113) Dess-Martin reagent (0.34 g, 0.80 mmol) was added to Compound 94 (prepared in Example 28) (0.31 g, 0.664 mmol) in CH2Cl2 (12 mL). The mixture was stirred at rt for 3 h, then another portion of Dess-Martin reagent (50 mg, 0.12 mmol) was added and the mixture was stirred for 1 h until TLC showed that Compound 94 was no longer present. The reaction was quenched with 25% Na2S2O3 in water (3 mL). The aqueous layer was extracted with chloroform several times. The organic layers were combined and washed with water and brine, then dried (Na2SO4) and evaporated in vacuo to a reddish solid. The solid was purified by flash column chromatography (95:5:0.5; DCM:MeOH:NH4OH) to afford Compound 112 (300 mg, 98%) as a red solid. 1H NMR (CD3OD) δ9.43 (s, 1H), 8.84 (d, J=2.4 Hz, 1H), 8.65 (d, J=4.8 Hz, 1H), 8.26 (s, 1H), 8.12 (m, 1H), 7.70 (m, 1H), 7.61 (m, 2H), 7.43 (m, 2H), 7.14 (m, 2H), 6.75 (t, J=7.5 Hz, 1H), 6.43 (d, J=8.1 Hz, 1H), 4.46 (t, J=6.9 Hz, 2H), 1.96 (m, 2H). ES-MS m/z 462 (MH+).
References:
US2003/55097,2003,A1
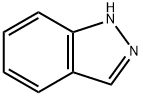
271-44-3
249 suppliers
$6.00/250mg

247128-24-1
9 suppliers
$378.00/1g
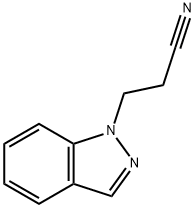
23300-94-9
9 suppliers
inquiry

247128-24-1
9 suppliers
$378.00/1g