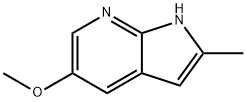
1H-Pyrrolo[2,3-b]pyridine, 5-methoxy-2-methyl- synthesis
- Product Name:1H-Pyrrolo[2,3-b]pyridine, 5-methoxy-2-methyl-
- CAS Number:397842-91-0
- Molecular formula:C9H10N2O
- Molecular Weight:162.19
![1H-Pyrrolo[2,3-b]pyridine, 5-Methoxy-2-Methyl-1-[(4-Methylphenyl)sulfonyl]-](/CAS/GIF/651744-20-6.gif)
651744-20-6
0 suppliers
inquiry
![1H-Pyrrolo[2,3-b]pyridine, 5-methoxy-2-methyl-](/CAS/GIF/397842-91-0.gif)
397842-91-0
12 suppliers
inquiry
Yield:397842-91-0 94%
Reaction Conditions:
with water;sodium hydroxide in methanol at 80; for 0.5 h;
Steps:
2-(l-(4-chlorobenzyl)-5-methoxy-2-methyl-lH-pyrrolo[2,3-b]pyridin-3-yl)-N-(2- methoxypyridin-4-yl)-2-oxoacetamide (38); [00424] To a solution of 5-bromo-lH-pyrrolo[2,3-b]pyridine (2.00 g, 10.2 mmol) inN,N-dimethylformamide (30 mL) was added a 25% weight solution in methanol of sodium methoxide (46.4 mL, 203 mmol) followed by copper(I) bromide (2.91 g, 20.3 mmol). The reaction mixture was heated at 140 °C for 2.5 hours, after it was cooled to room temperature and concentrated to remove most of the NN-dimethylformamide. Water (100 mL) was added followed by saturated aqueous sodium bicarbonate solution (20 mL). The mixture was extracted with EtOAc (3 x 50 mL), dried (magnesium sulfate), filtered and concentrated to a residue. Purification was achieved by silica gel chromatography (Biotage) using using 0 to 50% ethyl acetate in hexanes over 60 minutes to afford 5-methoxy-lH-pyrrolo[2,3-b]pyridine(0.680 g, 4.59 mmol, 45%yield) as a green solid. NMR (400 MHz, CDC13) δ (ppm): 9.90 (br. s, 1H), 8.10 (m, 1H), 7.46 (m, 1H), 7.33 (m, 1H), 6.44 (m, 1H), 3.89 (s, 3H).[00425] To a mixture of 5-methoxy-lH-pyrrolo[2,3-b]pyridine (670 mg, 4.52 mmol), and benzyltributylammonium bromide (70.5 mg, 0.226 mmol) in dichloromethane (20 mL) was added powdered sodium hydroxide (561 mg, 14.0 mmol). The reaction mixture was cooled to 0 °C, after which 4-methylbenzene-l-sulfonyl chloride (991 mg, 5.20 mmol) was added portionwise. The mixture was stirred at 0 °C for 15 min then warmed to room temperature where it was stirred for two hours, extracted with toluene (2 x 50 mL), dried (sodium sulfate), filtered and concentrated. The crude product was triturated in ether and filtrated to afford the compound 5-methoxy-l-tosyl-lH-pyrrolo[2,3-b]pyridine (1.21 g, 4.01 mmol, 89% yield) as a solid. 1H NMR (400 MHz, CDC13) δ (ppm): 8.15 (s, 1H), 8.05 (m, 2H), 7.66 (m, 1H), 7.25 - 7.30 (m, 3H), 6.51 (m, 1H), 3.83 (s, 3H), 2.35 (s, 3H).[00426] To a -60 °C solution of 5-methoxy-l-tosyl-lH-pyrrolo[2,3-b]pyridine (1.20 g, 3.97 mmol) in tetrahydrofuran (25 mL) was added 2M intetrahydrofuran/ethylbenzene/toluene solution of lithium diisopropyl amide (3.97 mL, 7.94 mmol). The reaction mixture was stirred at -60 °C for 30 minutes, after which iodomethane (0.298 mL, 4.76 mmol) was added, after which the reaction mixture was room temperature. Upon warming, the reaction was poured into ice water, extracted with ethyl acetate (3 x 50 mL), washed with water (3 x 50 mL), saturated sodium chloride solution (3 x 50 mL), dried (magnesium sulfate), filtered and concentrated. Purification was achieved by silica gel chromatography (Biotage) using 0 to 50% ethyl acetate in hexanes to afford 5-methoxy-2- methyl-l-tosyl-lH-pyrrolo[2,3-b]pyridine (0.550 g, 1.74 mmol, 44 % yield). NMR (400 MHz, CDC13) δ (ppm): 8.08 (s, 1H), 7.96 (d, 2H), 7.26 (m, 2H), 7.16 (m, 1H), 6.20 (s, 1H), 3.83 (s, 3H), 2.69 (s, 3H), 2.35 (s, 3H).[00427] A solution of 5-methoxy-2-methyl-l-tosyl-lH-pyrrolo[2,3-b]pyridine (1.14 g, 3.60 mmol) and sodium hydroxide (14.4 g, 360 mmol) in methanol (70 mL) and water (70 mL) was heated at 80 °C for 30 minutes, after which it was cooled to room temperature, poured into to ice water, extracted with ethyl acetate (3 x 50 mL), washed with saturated sodium chloride solution (3 x 50 mL), dried (magnesium sulfate), filtered and concentrated to afford 5-methoxy-2-methyl-lH-pyrrolo[2,3-b]pyridine (0.550 g, 3.39 mmol, 94 % yield) as a solid. NMR (400 MHz, CDC13) δ (ppm): 9.48 (b, 1H), 7.97 (m,lH), 7.33 (m, 1H), 6.10 (m? 1H), 3.89 (s, 3H) 2.49 (s, 3H). This material was used in the subsequent step without any purification.[00428] 2-(l-(4-chlorobenzyl)-5-methoxy-2-methyl-lH-pyrrolo[2,3-b]pyridin-3-yl)-N- (2-methoxypyridin-4-yl)-2-oxoacetamide was synthesized as a yellow solid in 55% yield starting from l-(4-chlorobenzyl)-5-methoxy-2-methyl-lH-pyrrolo[2,3-b]pyridin using general procedure D. NMR (400 MHz, CDC13) δ (ppm): 9.13 (br. s, 1H), 8.10 - 8.17 (m, 3H), 7.25 - 7.27 (m, 3H), 7.14 (d, 1H), 7.06 (m, 2H), 5.55 (s, 2H), 3.96 (s, 3H), 3.93 (s, 3H), 2.70 (s, 3H).[00429] 2-(l-(4-chlorobenzyl)-5-methoxy-2-methyl-lH-pyrrolo[3,2-b]pyridin-3-yl)-N- (2-methoxypyridin-4-yl)-2-oxoacetamide was synthesized as a solid starting from methoxy-2- methyl-lH-pyrrolo[3,2-b]pyridine using general route 2. NMR (400 MHz, CD3OD) δ (ppm): 8.02 (d, 1H), 7.69 (d, 1H), 7.33 (m, 3H), 7.21 - 7.23 (m, 1H), 7.06 (d, 2H), 6.56 (d, 1H), 5.51 (s, 2H), 3.90 (s, 3H), 3.52 (s, 3H), 2.81 (s, 3H).
References:
WO2012/88469,2012,A1 Location in patent:Page/Page column 175-177