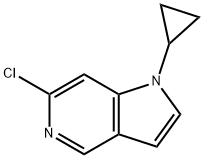
1H-Pyrrolo[3,2-c]pyridine, 6-chloro-1-cyclopropyl- synthesis
- Product Name:1H-Pyrrolo[3,2-c]pyridine, 6-chloro-1-cyclopropyl-
- CAS Number:1324003-17-9
- Molecular formula:C10H9ClN2
- Molecular Weight:192.64
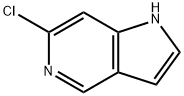
74976-31-1
177 suppliers
$9.00/100mg
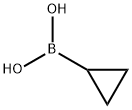
411235-57-9
432 suppliers
$6.00/1g
![1H-Pyrrolo[3,2-c]pyridine, 6-chloro-1-cyclopropyl-](/CAS/20210111/GIF/1324003-17-9.gif)
1324003-17-9
5 suppliers
inquiry
Yield:1324003-17-9 57%
Reaction Conditions:
with sodium carbonate;[2,2]bipyridinyl;copper diacetate in 1,2-dichloro-ethane at 70;
Steps:
7.A.7A
Example 7: Preparation of Intermediates 7; [0370] Step A: 7A In a 2L round bottom flask were added copper(II) acetate (8.9 g, 49 mmol) and 2,2'-bipyridine (7.8 g, 49 mmol) in 1 ,2-dichloroethane (240 ml). This was heated to 70 °C. Separately were suspended 6-chloro-lH-pyrrolo[3,2-c]pyridine (15 g, 98 mmol) and cyclopropylboronic acid (17 g, 200 mmol) in 1 ,2-dichloroethane (240 ml). To the first heated mixture was added sodium carbonate (21 g, 200 mmol), followed by the second mixture, and the resulting mixture turned from green to dark red color. The mixture was heated to 70 °C overnight. Next day, conversion was -50%, and another 0.25 equivalent of Cu(OAc)2 (4.5g), 0.25 equivalent of BiPy (3.9g), and 1.0 equivalent of cyclopropylboronic acid (8.5g) were added. The mixture was continued to stir at 70 °C for 12 hr. After cooling to room temperature, 250ml brine and 250 ml EtOAc were added. The layers were separated, and the aqueous layer was extracted twice with EtOAc (2 x 150ml). Copper salt was filtered from organic layers, which were then dried over sodium sulfate, filtered, and concentrated to obtain the crude product as a thick black oil. Dissolved in MeOH, the crude product was loaded onto silica before silica column chromatography. Collected fractions were pooled and concentrated to give a thick yellow oil containing bi- pyridine. To this oil were added 100ml EtOAc and 100ml saturated aqueous CuS04 solution. The layers were separated, and the aqueous layer was separated to remove blue copper salt. The filtrate was then extracted twice with EtOAc (2 x 50ml). Combined organic layers were dried with sodium sulfate, filtered and concentrated to obtain the product 6-chloro-l-cyclopropyl-lH-pyrrolo[3,2-c]pyridine (7A, 11 g, 57%) as a light yellow solid.
References:
WO2011/97079,2011,A1 Location in patent:Page/Page column 96-97