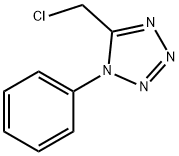
1H-Tetrazole,5-(chloromethyl)-1-phenyl-(9CI) synthesis
- Product Name:1H-Tetrazole,5-(chloromethyl)-1-phenyl-(9CI)
- CAS Number:64473-40-1
- Molecular formula:C8H7ClN4
- Molecular Weight:194.62
587-65-5
134 suppliers
$13.00/1g

64473-40-1
17 suppliers
$45.00/2.5g
Yield:64473-40-1 95%
Reaction Conditions:
Stage #1: N-chloroacetyl-anilinewith phosphorus pentachloride in benzene at 50 - 60;
Stage #2: with hydrogen azide in benzene at 5 - 10; for 1 h;Inert atmosphere;Reflux;
Steps:
Synthesis of 1-aryl-5-(chloromethyl)-1H-tetrazoles 5a-f
General procedure: Finely ground PCl5 (35.0 g, 0.17 mol) was added to a suspension of chloroacetic acid arylamide (0.15 mol) in PhH (150 ml). The reaction mixture was stirred protected from atmospheric moisture until evolution of HCl ceased, gradually increasing the temperature to 50-60°C. The mixture was then cooled to 5-10°C, and a solution of HN3 (0.25-0.30 mol) in PhH (150 ml) was added in small portions with stirring.15 The reaction mixture was slowly heated to boiling with stirring,maintaining a moderate evolution of HCl, then heated under reflux for 1 h, cooled, and poured into a mixture of water and ice (250-300 ml). After the ice melted, the formed two-phase mixture was heated under reflux for 30 min with vigorous stirring to decompose POCl3. After the reaction mixture was cooled to room temperature, the organic layer was separated and washed with H2O (2×100 ml). The solvent was evaporated under reduced pressure, and the residue was crystallized from CCl4. Compound 5b was obtained as an oil, which was used in the next step without additional purification. 5-(Chloromethyl)-1-phenyl-1H-tetrazole (5). Yield 27.8 g (95%), colorless compact crystals, mp 75-76°C (mp 76-77°C14). 1H NMR spectrum, δ, ppm: 5.00 (2H, s, CH2); 7.63-7.71 (5H, m, H Ph).
References:
Koltsov, Nikolai Yu. [Chemistry of Heterocyclic Compounds,2019,vol. 55,# 8,p. 768 - 772][Khim. Geterotsikl. Soedin.,2019,vol. 55,# 8,p. 768 - 772,5]

62-53-3
653 suppliers
$10.00/1g

64473-40-1
17 suppliers
$45.00/2.5g
874483-75-7
0 suppliers
inquiry

587-65-5
134 suppliers
$13.00/1g
71-43-2
690 suppliers
$11.19/25ML

64473-40-1
17 suppliers
$45.00/2.5g
