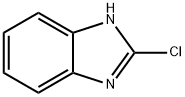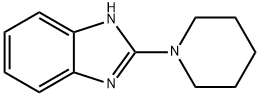
2-(1-piperidinyl)-1H-benzimidazole(SALTDATA: FREE) synthesis
- Product Name:2-(1-piperidinyl)-1H-benzimidazole(SALTDATA: FREE)
- CAS Number:2851-12-9
- Molecular formula:C12H15N3
- Molecular Weight:201.27
Yield:2851-12-9 88%
Reaction Conditions:
in i-Amyl alcohol at 150; for 2.5 h;Microwave irradiation;
Steps:
4.3. General procedure to synthesize compounds M084, 4-7 and 11-14
General procedure: A solution of 2-chloro-[1H]-benzimidazole (305 mg; 2 mmol) and appropriate amine (12 mmol) in methyl-1-butanol (4.0 mL) was irradiated in a microwave reactor at 150 °C (detected by IR infrared temperature sensor) for 2.5 h. The reaction mixture was concentrated under reduced pressure at 50 °C and the crude product was further purified by silica chromatography (200-300 mesh, eluting with petroleum ether/EtOAc = 2:1) to give the titled 2-aminobenzimidazoles in 65-95% yields.
References:
Zhu, Jinmei;Wu, Chun-Feng;Li, Xiaobing;Wu, Gui-Sheng;Xie, Shan;Hu, Qian-Nan;Deng, Zixin;Zhu, Michael X.;Luo, Huai-Rong;Hong, Xuechuan [Bioorganic and Medicinal Chemistry,2013,vol. 21,# 14,p. 4218 - 4224]
65069-94-5
7 suppliers
inquiry

2851-12-9
9 suppliers
$98.00/1g

31468-18-5
3 suppliers
inquiry

2851-12-9
9 suppliers
$98.00/1g
57159-81-6
25 suppliers
inquiry

2851-12-9
9 suppliers
$98.00/1g