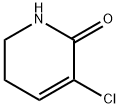
2(1H)-Pyridinone, 3-chloro-5,6-dihydro- synthesis
- Product Name:2(1H)-Pyridinone, 3-chloro-5,6-dihydro-
- CAS Number:207976-92-9
- Molecular formula:C5H6ClNO
- Molecular Weight:131.56

41419-12-9
8 suppliers
inquiry

207976-92-9
20 suppliers
$350.00/50mg
Yield:207976-92-9 90%
Reaction Conditions:
with lithium carbonate;lithium chloride in N,N-dimethyl-formamide at 130; for 4 h;
Steps:
3-Chloro-5,6-dihydropyridin-2(1H)-one (11)
DMF (1.35 dm3), 90 g 10 (0.536 mol), 79.2 g Li2CO3(1.071 mol), and 22.7 g LiCl (0.536 mol) were sequentially charged at RT into a 3 dm3 three-necked round-bottomed flask, fitted with a condenser, a mechanical stirrer, and aCaCl2 guard tube. The reaction mixture was then stirred at 130 °C for 4 h, cooled to RT and poured into 2.70 dm3 ice cold water. The resulting suspension was filtered through aCelite bed and the residue washed with 900 cm3 dichloromethane.The combined filtrate was extracted with dichloromethane (3 9 1.80 dm3). The organic layers were combined, dried over anhydrous Na2SO4, concentrated under reduced pressure, and finally dried under high vacuumat 60 °C for 1 h to obtain 11 (63.6 g, 90%, 97.5% purity byHPLC analysis) as a dark brown solid. 1HNMR spectral dataof 11 (see Supplementary Material for details) was found tobe consistent with the values reported in Ref. [14].
References:
Nevuluri, Narasimha Rao;Rapolu, Rajesh Kumar;Iqbal, Javed;Kandagatla, Bhaskar;Sen, Saikat;Dahanukar, Vilas H.;Oruganti, Srinivas [Monatshefte fur Chemie,2017,vol. 148,# 8,p. 1477 - 1482]
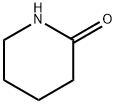
675-20-7
353 suppliers
$5.00/1g

207976-92-9
20 suppliers
$350.00/50mg
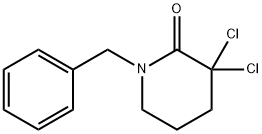
305839-60-5
0 suppliers
inquiry

207976-92-9
20 suppliers
$350.00/50mg
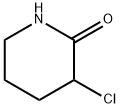
16834-22-3
4 suppliers
inquiry

207976-92-9
20 suppliers
$350.00/50mg