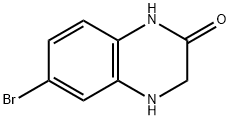
2(1H)-Quinoxalinone, 6-broMo-3,4-dihydro- synthesis
- Product Name:2(1H)-Quinoxalinone, 6-broMo-3,4-dihydro-
- CAS Number:854584-01-3
- Molecular formula:C8H7BrN2O
- Molecular Weight:227.06
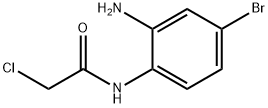
854583-92-9
7 suppliers
inquiry

854584-01-3
54 suppliers
$65.00/60mg
Yield: 34%
Reaction Conditions:
with sodium carbonate;sodium iodide in acetonitrileReflux;Inert atmosphere;
Steps:
18
A mixture of N-(2-amino-4-bromophenyl)-2-chloroacetamide (4.5 g, 17.0 mmol), sodium iodide (101 mg, 2.0 mmol) and sodium carbonate (3.6 g, 33.8 mmol) in 300 ml acetonitrile was refluxed overnight under an atmosphere of nitrogen. Then the acetonitrile was removed under vacuum. The resulting solid was suspended in 150 ml water acidified to pH 5-6 with 1 N HCl. Extraction with ethyl acetate, washing of the combined organic layers with brine, drying over MgSO4, removing the solvent under reduced pressure and purification of the crude product by flash chromatography on silica gel (petroleum ether/ethyl acetate, 1.5/1) yielded 6-bromo-3,4-dihydroquinoxalin-2(1H)-one (1.3 g, 5.8 mmol, 34%) as yellow solid.
References:
Universitat des Saarlandes US2011/112067, 2011, A1 Location in patent:Page/Page column 33
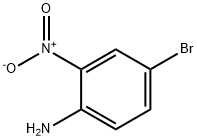
875-51-4
308 suppliers
$5.00/1g

854584-01-3
54 suppliers
$65.00/60mg
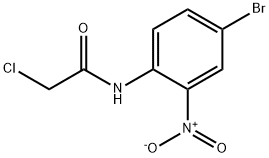
854583-85-0
14 suppliers
$251.00/1g

854584-01-3
54 suppliers
$65.00/60mg

321-23-3
256 suppliers
$10.00/1g

854584-01-3
54 suppliers
$65.00/60mg