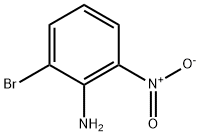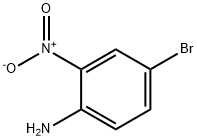
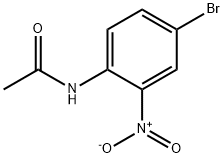
4-Bromo-2-nitroaniline synthesis
- Product Name:4-Bromo-2-nitroaniline
- CAS Number:875-51-4
- Molecular formula:C6H5BrN2O2
- Molecular Weight:217.02

75-09-2
1197 suppliers
$10.00/25 mL
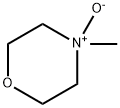
7529-22-8
382 suppliers
$5.00/1g
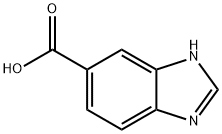
15788-16-6
268 suppliers
$5.00/250mg

875-51-4
308 suppliers
$5.00/1g
58442-17-4
92 suppliers
$42.00/100mg
Yield:875-51-4 452 mg (32%, 2 steps)
Reaction Conditions:
with LiAlH4;tetrapropylammonium perruthennate;silica gel in tetrahydrofuran;N,N-dimethyl-formamide
Steps:
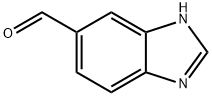
5 5-Formylbenzimidazole (18)
EXAMPLE 5 5-Formylbenzimidazole (18) A suspension of 5-benzimidazolecarboxylic acid (1.57 g, 9.7 mmol) in dry THF (50 ml) was cooled to -78° C. under N2, and treated with LiAlH4 (736 mg, 19.4 mmol). After the addition, the mixture was allowed to warm slowly to room temperature and then stirred at r.t. overnight. The mixture was quenched by MeOH and H2 O cautiously, and passed through a short silica gel column eluding with 10% MeOH/EtOAc. The elude was concentrated to give 876 mg crude alcohol as a solid. The crude alcohol (876 mg) was dissolved in a mixture of DMF (3 ml), THF (10 ml) and CH2 Cl2 (40 ml). 4-Methylmorpholine N-oxide (2.25 g, 19.2 mmol), 4A molecular sieves (5 g), and TPAP (169 mg, 0.48 mmol) were subsequently added to the crude alcohol solution. The mixture was stirred at room temperature overnight, and filtered through a pad of silica gel eluding with 10% MeOH/EtOAc. The elute was concentrated and further purified by flash chromatography on silica gel eluding with 0-10% MeOH/EtOAc to give 452 mg (32%, 2 steps) of 17 as a white solid: mp 164°-166° C.; IR KBr) 3087, 2818, 1690, 1292; 1 H NMR (CD3 OD) δ 9.95 (1H, s), 8.34 (1H, s), 8.08 (1H, d, J=1.5), 7.74 (1H, dd, J=8.4, 1.5), 7.63 (1H, d, J=8.4); 13 C NMR (CD3 OD) δ 194.2, 146.0, 143.0, 139.8, 133.6, 124.9, 120.7, 116.6; Anal. Calcd for C8 H6 N2 O: C, 65.75; H, 4.14; N, 19.17. Found: C, 65.60; H, 4.17; N, 19.08.
References:
Rutgers, The State University of New Jersey US5767142, 1998, A

88-74-4
8 suppliers
$10.00/1g

875-51-4
308 suppliers
$5.00/1g
881-50-5
62 suppliers
$8.00/100mg

875-51-4
308 suppliers
$5.00/1g

106-40-1
458 suppliers
$12.00/5g

875-51-4
308 suppliers
$5.00/1g