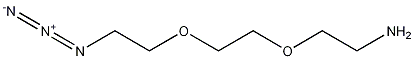
2-[2-(2-Azidoethoxy)ethoxy]ethanamine synthesis
- Product Name:2-[2-(2-Azidoethoxy)ethoxy]ethanamine
- CAS Number:166388-57-4
- Molecular formula:C6H14N4O2
- Molecular Weight:174.2
![synthesis of 2-[2-(2-Azidoethoxy)ethoxy]ethanamine synthesis of 2-[2-(2-Azidoethoxy)ethoxy]ethanamine](/NewsImg/2022-10-12/6380118429396640864122844.jpg)
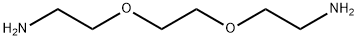
929-59-9
227 suppliers
$8.00/5g
![2-[2-(2-Azidoethoxy)ethoxy]ethanamine](/CAS2/GIF/166388-57-4.gif)
166388-57-4
111 suppliers
inquiry
Yield:166388-57-4 78%
Reaction Conditions:
with sodium azide;copper(ll) sulfate pentahydrate;trifluoromethylsulfonic anhydride;potassium carbonate in methanol;dichloromethane;water at 20;
Steps:
2-(2-(2-Azidoethoxy-exthoxy)ethanamine (N3-PEG-NH2)
NaN3 (3.44 g, 52.9 mmol) in H2O (12.0 mL) was mixed with CH2Cl2 (10.0 mL) in an ice-water bath. Tf2O (1.8 mL, 10.7 mmol) in CH2Cl2 (8.0 mL) was added drop wise to the solution for 1 hr at 0 oC.1 The resulting mixture was stirred at the same temperature for an additional 4 hr, and the layers were separated. The aqueous layer was further extracted with CH2Cl2 (40 mL). The CH2Cl2 layers were combined, washed with aqueous saturated Na2CO3 (30 mL) and used as is without further purification. 2,2'-(Ethylenedioxy) bis(ethylamine) (3.1 mL, 21.2 mmol) in MeOH (20 mL) was added to K2CO3 (1.70 g, 12.3 mmol) and CuSO45H2O (cat. 14 mg) in H2O (4 mL). The CH2Cl2 extracts of the first reaction was added drop wise over a period of 5 hr, and the reaction mixture was stirred at rt for 36 hr. Then the layers were separated and the organic layer was dried over anhydrous Na2SO4. The solution was filtered and concentrated under vacuum. The residue was purified by silica gel flash chromatography (MeOH:CH2Cl2 5:95) to afford the desired product as a light yellow oil (Rf = 0.24 in 1:4 MeOH:CH2Cl2). Yield: 1.44 g, 78%. 1H NMR (400 MHz, CDCl3, rt): δ(ppm) 2.94 (s, br, 2 H), 3.41 (t, J = 5.00 Hz, 2 H), 3.56 (t, J = 5.04 Hz, 2 H), 3.66 (m, 6 H), 3.89 (s, br, 2 H); 13C NMR (100.6 MHz, CDCl3, rt): δ(ppm) 42.02, 51.63, 70.95, 71.17, 71.60, 72.65; ESI-LRMS: [M+H]+, 175.4 (100%).
References:
Li, Ying;Schaffer, Paul;Perrin, David M. [Bioorganic and Medicinal Chemistry Letters,2013,vol. 23,# 23,p. 6313 - 6316] Location in patent:supporting information
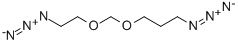
59559-06-7
56 suppliers
$65.00/50 mg
![2-[2-(2-Azidoethoxy)ethoxy]ethanamine](/CAS2/GIF/166388-57-4.gif)
166388-57-4
111 suppliers
inquiry

950683-55-3
61 suppliers
$60.00/100mg
![2-[2-(2-Azidoethoxy)ethoxy]ethanamine](/CAS2/GIF/166388-57-4.gif)
166388-57-4
111 suppliers
inquiry

112-27-6
783 suppliers
$6.00/100g
![2-[2-(2-Azidoethoxy)ethoxy]ethanamine](/CAS2/GIF/166388-57-4.gif)
166388-57-4
111 suppliers
inquiry
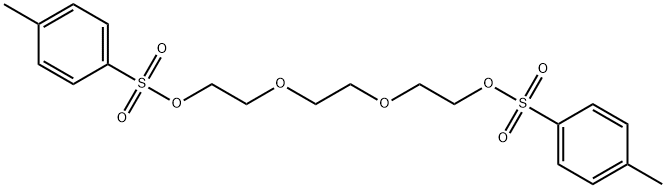
19249-03-7
239 suppliers
$9.00/1g
![2-[2-(2-Azidoethoxy)ethoxy]ethanamine](/CAS2/GIF/166388-57-4.gif)
166388-57-4
111 suppliers
inquiry