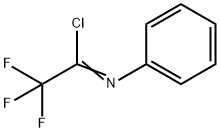
2,2,2-Trifluoro-N-phenylacetimidoyl Chloride synthesis
- Product Name:2,2,2-Trifluoro-N-phenylacetimidoyl Chloride
- CAS Number:61881-19-4
- Molecular formula:C8H5ClF3N
- Molecular Weight:207.58
404-24-0
98 suppliers
$15.00/500mg

61881-19-4
77 suppliers
$23.00/250mg
Yield:61881-19-4 61%
Reaction Conditions:
with triethylamine;dichlorotriphenylphosphorane in acetonitrile; for 3 h;Reflux;
Steps:
1.1. N-Phenyl-2,2,2-trifluoroacetimidoyl chloride (PTFAI-Cl)
N-Phenyltrifluoroacetamide [18] (4.00 g, 21.2 mmol) anddichlorotriphenylphosphorane (17.6 g, 52.9 mmol) were suspendedin MeCN (80 ml) and Et3N (7.67 ml, 55.0 mmol) was added. Thesuspension was refluxed for 3 h and then cooled to 0 C. Theprecipitated solids (Et3N.HCl and Ph3PO) were removed by filtrationand the filtrate concentrated under reduced pressure. The residue was dissolved in minimal CHCl3 and triturated with hexanes.The precipitate was removed by filtration and the filtrateconcentrated under reduced pressure. The residue was purified byflash chromatography (Et2O/hexanes 1:4) afford the chloride as acolourless liquid (2.68 g, 61%). 1H NMR (500 MHz, CDCl3)d 7.48e7.40 (m, 2H), 7.34e7.28 (m, 1H), 7.13e7.07 (m, 2H); 13C NMR(125 MHz, CDCl3) d 143.6, 132.1 (q, J 277.1 Hz), 129.3, 127.6, 120.8,117.0 (q, J 43.0 Hz); IR (acetone) 1506, 1260, 1093, 1055, 1012, 795,693 cm1; HRMS (ESI) calcd for C8H6ClF3N [MH] 208.0135,found 208.0136.
References:
Smith, Dylan G.M.;Williams, Spencer J. [Carbohydrate Research,2017,vol. 450,p. 10 - 11]

62-53-3
637 suppliers
$10.00/1g

61881-19-4
77 suppliers
$23.00/250mg