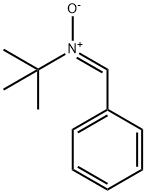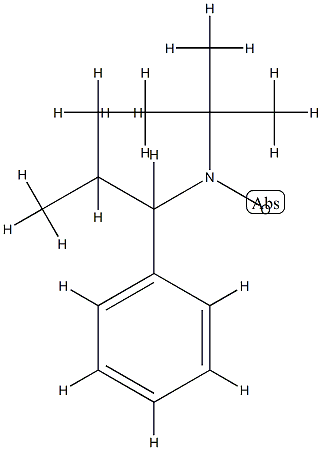
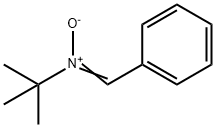
2,2,5-TriMethyl-4-phenyl-3-azahexane-3-nitroxide synthesis
- Product Name:2,2,5-TriMethyl-4-phenyl-3-azahexane-3-nitroxide
- CAS Number:61015-94-9
- Molecular formula:C14H22NO*
- Molecular Weight:220.3306
3376-24-7
137 suppliers
$28.00/250MG
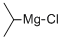
1068-55-9
319 suppliers
$20.00/25ml

61015-94-9
3 suppliers
$629.29/250MG
Yield:61015-94-9 77%
Reaction Conditions:
Stage #1: N-tert-butyl-α-phenylnitrone;isopropylmagnesium chloride in tetrahydrofuran at -78 - 20;Inert atmosphere;
Stage #2: with ammonium hydroxide;copper diacetate in methanol;
Steps:
2-7. Synthesis of N-tert-butyl-N-(1-diethylphosphono-2,2-dimethylpropyl) nitroxide (TIPNO)
A solution of isopropyl magnesium chloride solution (3.0 mL, 2.3 M in THF) was dropwiselyadded to a solution of the oxide (0.40 g, 2.2 mmol) in THF (10 mL) at -78 °C over 1 h under nitrogenatmosphere, and the resulting solution was stirred for overnight at room temperature. A saturatedaqueous NH4Cl solution (10 mL) was added to the mixture, and the aqueous phase was extracted withEt2O. The combined organic phase was washed with saturated brine and dried over MgSO4. Thesolvent was removed under reduced pressure, and the residual crude product was dissolved intomethanol (10 mL) containing copper(II) acetate (22 mg, 0.15 mol) and concentrated aqueous ammoniasolution (1.0 mL). After a stream of air was bubbled through the solution for 3 h, the mixture wastreated with a saturated aqueous NaHSO3 solution (10 mL) and extracted with CHCl3. The combinedorganic phase was dried over MgSO4, and was removed under reduced pressure. The residue waspurified by flash chromatography on silica gel using hexane:ethyl acetate (15:1 v/v) as an eluent toafford the title product (0.31 g, 77% yield) as orange powder.
References:
Li, Xiaopei;Kato, Tatsuhisa;Nakamura, Yasuyuki;Yamago, Shigeru [Bulletin of the Chemical Society of Japan,2021,vol. 94,# 3,p. 966 - 972] Location in patent:supporting information

3376-24-7
137 suppliers
$28.00/250MG
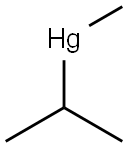
29138-88-3
0 suppliers
inquiry

61015-94-9
3 suppliers
$629.29/250MG
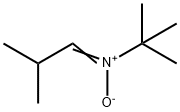
85664-55-7
1 suppliers
inquiry
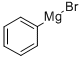
100-58-3
183 suppliers
$18.00/10ml

61015-94-9
3 suppliers
$629.29/250MG

100-52-7
945 suppliers
$20.37/250g

61015-94-9
3 suppliers
$629.29/250MG