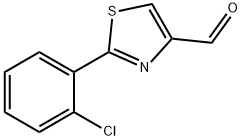
2-(2-CHLORO-PHENYL)-THIAZOLE-4-CARBALDEHYDE synthesis
- Product Name:2-(2-CHLORO-PHENYL)-THIAZOLE-4-CARBALDEHYDE
- CAS Number:639517-84-3
- Molecular formula:C10H6ClNOS
- Molecular Weight:223.68
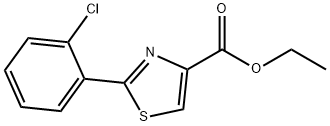
132089-36-2
38 suppliers
$55.00/250mg

639517-84-3
30 suppliers
$45.00/10mg
![[2-(2-CHLORO-PHENYL)-THIAZOL-4-YL]-METHANOL](/CAS/GIF/639517-86-5.gif)
639517-86-5
24 suppliers
inquiry
Yield:-
Reaction Conditions:
with diisobutylaluminium hydride in dichloromethane;toluene at -78; for 1 h;
Steps:
3.d
d) To a solution of ethyl 2- (2-chlorophenyl)-4-thiazolecarboxylate (1.34 g, [5.] 0 mmol), prepared as described above, in [CH2CI2] (20 mL) at-78 °C is added diisobutylaluminum hydride (10.5 mL of 1.0 M solution in toluene, 10.5 mmol) and the resulting reaction mixture is stirred at-78 °C for 1 hour after which time sodium fluoride (1.5 g) and water (0.5 mL) are added and the reaction mixture is allowed to warm to room temperature and filtered. The filtrate is concentrated to provide the crude reduction product which is purified by column chromatography (10% ethyl acetate in hexanes) to afford 138 mg of [[2- (2-CHLOROPHENYL)-1,] 3-thiazol-4-yl] carboxyaldehyde and 605 mg of [[2- (2-CHLOROPHENYL)-1,] 3-thiazol-4-yl] methanol. The of [[2-(2-CHLOROPHENYL)-1, 3-THIAZOL-4-YL]] carboxyaldehyde portion is then used for subsequent steps while the [[2-(2-CHLOROPHENYL)-1,] 3-thiazol-4-yl] methanol portion is converted to [[2-(2-CHLOROPHENYL)-1, 3-THIAZOL-4-YL]] carboxyaldehyde via standard oxidation conditions.
References:
WO2004/2977,2004,A1 Location in patent:Page 23-24
![[2-(2-CHLORO-PHENYL)-THIAZOL-4-YL]-METHANOL](/CAS/GIF/639517-86-5.gif)
639517-86-5
24 suppliers
inquiry

639517-84-3
30 suppliers
$45.00/10mg
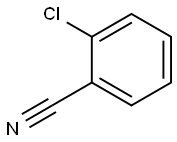
873-32-5
545 suppliers
$9.00/1g

639517-84-3
30 suppliers
$45.00/10mg
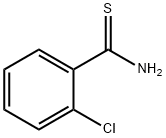
15717-17-6
77 suppliers
$19.00/1g

639517-84-3
30 suppliers
$45.00/10mg