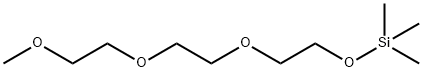
2,2-dimethyl-3,6,9,12-tetraoxa-2-silatridecane synthesis
- Product Name:2,2-dimethyl-3,6,9,12-tetraoxa-2-silatridecane
- CAS Number:864079-62-9
- Molecular formula:C10H24O4Si
- Molecular Weight:236.38
Yield:864079-62-9 73%
Reaction Conditions:
with amberlyst-15 at 50; for 3.5 h;Industrial scale;
Steps:
2 Preparation Detail for 2,2-dimethyl-3,6,9,12-tetraoxa-2-silatridecane
EXAMPLE 2
Preparation Detail for 2,2-dimethyl-3,6,9,12-tetraoxa-2-silatridecane
A glass reactor (20 L, jacketed, Chemglass) equipped with drain valve, internal temperature probe, reflux condenser, gas inlet/outlet adapters and powder port was flushed with nitrogen.
The jacket of the reactor was connected to a Huber 430 heating/chilling circulator.
The gas outlet port was connected to a scrubber consisting of about 4 L water in a polypropylene drum.
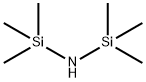
The reactor was charged with triethylene glycol monomethyl ether (3196.4 g, 19.47 mol, 1.0 eq, TCI lot FGI01) and 1,1,1,3,3,3-hexamethyldisilazane (2199.7 g, 13.6 mol, 0.07 eq, Alfa lot H09W015 (1935.4 g) and lot 10151582 (264.3 g)).
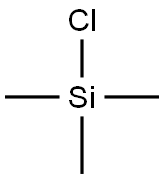
Add Amberlyst-15 (319.6 g, 10 wt %, Aldrich lot MKBD4929).
The stirring speed was set to 80-95 rpm to keep the catalyst suspended.
The circulator was set to control the process (internal reaction mixture) by slowly ramping the temperature up to 50° C.
The reaction was followed by GC/MS (Agilent HP-5MS, 0.25 um, 30 m*0.250 mm, 30 deg/min).
The first sample was taken at 1 hour after reacting temperature and indicated complete consumption of starting triethylene glycol monomethyl ether.
The reaction was held at 50° C. for a total of 3.5 hours.
The reaction mixture was cooled to 20 C.
The contents of the reactor were drained onto a glass frit (medium porosity).
The filtrate was concentrated under reduced pressure on a Buchi rotary evaporator (R-215, bath temperature 55 C, pressure 1-3 mbar).
The HMDS was recovered (274 g).
The remaining concentrate weighed 4614 g.
The 5 L reactor was cleaned and charged with most of the concentrate (3033 g).
The material was fractionally distilled using a 5 plate Oldershaw column.
Once initial fractions were taken, the remainder of the crude material (and off-fractions) were added back and re-distilled.
The reactor jacket temperature was set to 125° C., the pressure was set to 3.5 mbar, and the condenser temperature was set to 15° C.
The distillation temperature measured at the top of the column was 107-109 for pure fractions.
Yield 3357 g (73%) of 2,2-dimethyl-3,6,9,12-tetraoxa-2-silatridecane (ANL-1NM3).
Purity 100% (average GC/MS peak area integration).
No other peaks were detected above baseline noise.
Moisture 26 ppm (per KF coulometric titration).
Flash point (closed cup) 112.5° C.
References:
US9263769,2016,B2 Location in patent:Page/Page column 6; 7