2-(2-METHYLPHENYL)ACETOPHENONE synthesis
- Product Name:2-(2-METHYLPHENYL)ACETOPHENONE
- CAS Number:5033-67-0
- Molecular formula:C15H14O
- Molecular Weight:210.27
Yield:5033-67-0 100%
Reaction Conditions:
with allylchloro-[1,3-bis(2,6-diisopropylphenyl)-4,5-dihydroimidazol-2-ylidene]palladium(II);sodium t-butanolate in tetrahydrofuran at 25 - 60; for 3.5 h;Inert atmosphere;
Steps:
I.1-1 Step 1-1
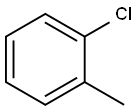
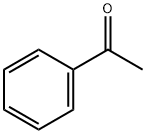
The product was prepared according to Org. Lett.2002, 4, 4053. To 24.0 g (0.250 mol) sodium t-butoxide in 200 ml water free THF, 12.0 g (0.100 mol) acetophe- none were added under argon at 25 °C.12.6 g (0.100 mmol) o-chlorotoluene were added. The reaction mixture was degassed with argon.287 mg (0.50 mmol) (SIPr)Pd(allyl)Cl (CAS: 478980- 01-7) were added and the reaction mixture was degassed with argon. The reaction mixture was stirred at 25 °C under argon for 2 h and then for 1.5 h at 60 °C. The reaction mixture was poured on water. The organic phase was extracted with dichloro- methane. The organic phase was dried with magnesium sulfate and the solvent was removed in vacuum. The product was used without purification for the next step. Yield 21.0 g (100 %)
References:
WO2022/107064,2022,A1 Location in patent:Page/Page column 128
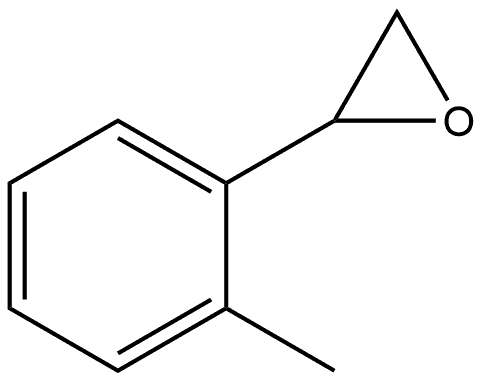
1195902-11-4
0 suppliers
inquiry
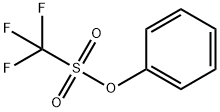
17763-67-6
87 suppliers
$13.00/1g

5033-67-0
11 suppliers
$215.00/1g

14309-60-5
19 suppliers
inquiry

5033-67-0
11 suppliers
$215.00/1g