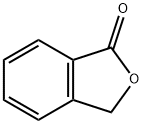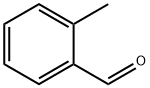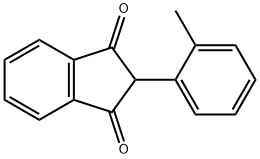
CHEMBRDG-BB 5303536 synthesis
- Product Name:CHEMBRDG-BB 5303536
- CAS Number:15432-97-0
- Molecular formula:C16H12O2
- Molecular Weight:236.27
Yield:15432-97-0 86%
Reaction Conditions:
with sodium methylate in methanol; for 2 h;Reflux;
Steps:
5.1.16 5.1.1.1 1,3-Dioxo-2-phenylindane (12)
General procedure: A solution of sodium methoxide in methanol (28%, 2.196 g, 11.4 mmol) was added to a suspension of benzaldehyde (307 mg, 2.89 mmol) and phthalide (387 mg, 2.89 mmol) in ethyl propionate (1.2 mL) at 30 °C, and the mixture was heated under reflux for 1 h. The reaction mixture was diluted with methanol and, was further heated for 1 h. After concentration in vacuo, water was added to the residue, and was washed with diethyl ether. After acidified with acetic acid, the suspension was stirred for 15 min. The product was collected by filtration, washed with water, and dried in vacuo to afford 12 (331 mg, 52%) as colorless powder.
References:
Inoue, Kazumi;Urushibara, Ko;Kanai, Misae;Yura, Kei;Fujii, Shinya;Ishigami-Yuasa, Mari;Hashimoto, Yuichi;Mori, Shuichi;Kawachi, Emiko;Matsumura, Mio;Hirano, Tomoya;Kagechika, Hiroyuki;Tanatani, Aya [European Journal of Medicinal Chemistry,2015,vol. 102,art. no. 8038,p. 310 - 319]

1807-49-4
0 suppliers
inquiry
108-88-3
7 suppliers
$16.10/100ml

15432-97-0
8 suppliers
$31.00/250mg
7561-48-0
8 suppliers
$28.60/25MG
![1(3H)-Isobenzofuranone, 3-[(2-methylphenyl)methylene]-](/CAS/20180601/GIF/19391-29-8.gif)
19391-29-8
0 suppliers
inquiry

124-41-4
683 suppliers
$12.00/25g

15432-97-0
8 suppliers
$31.00/250mg