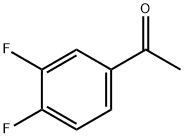
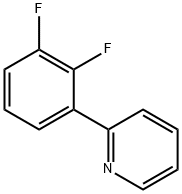
2-(3,4-DIFLUOROPHENYL)PYRIDINE synthesis
- Product Name:2-(3,4-DIFLUOROPHENYL)PYRIDINE
- CAS Number:387831-85-8
- Molecular formula:C11H7F2N
- Molecular Weight:191.18
Yield:387831-85-8 96.2%
Reaction Conditions:
with dmap;copper(II) ethylacetoacetate;C26H36NP;silver(I) acetate in propan-1-ol at 60; for 12 h;Reagent/catalyst;
Steps:
1 Example 1: 2-(3,4-Difluorophenyl)pyridine
To the appropriate amount of n-propanol in the reactor, 100 mmol of 3,4-difluorobenzoyl chloride, 100 mmol of n-butylamine, 5 mmol of copper(II) ethylacetoacetate, 5 mmol Ligand L1,100mmol dimethylaminopyridine 20mmol silver acetate and the mixture was stirred to warm to 60 , and stirring was continued at this temperature for 12 hours.
After the completion of the reaction, the reaction system was allowed to cool to room temperature, washed thoroughly with deionized water and the layers were separated. The organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The resulting residue was subjected to silica gel column chromatography,The eluent used was a mixture of ethyl acetate and n-butanol, both the volume ratio of 1: 3, i.e., of formula (I), 2- (3,4-difluorophenyl) pyridine, yield 96.2%.
References:
CN104529879,2016,B Location in patent:Paragraph 0045; 0046; 0047; 0048; 0049; 0050; 0051
109-04-6
533 suppliers
$6.00/25g
168267-41-2
344 suppliers
$14.00/5g

387831-85-8
8 suppliers
inquiry
98-98-6
637 suppliers
$5.00/10g

367-11-3
314 suppliers
$10.00/1g

387831-85-8
8 suppliers
inquiry
1101187-11-4
1 suppliers
inquiry