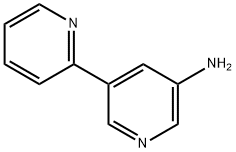
2,3'-bipyridin-5'-aMine synthesis
- Product Name:2,3'-bipyridin-5'-aMine
- CAS Number:1245745-55-4
- Molecular formula:C10H9N3
- Molecular Weight:171.2
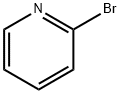
109-04-6
531 suppliers
$6.00/25g
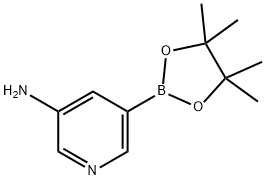
1073354-99-0
96 suppliers
$20.00/250mg

1245745-55-4
13 suppliers
inquiry
Yield:1245745-55-4 300 mg
Reaction Conditions:
with tetrakis(triphenylphosphine) palladium(0);caesium carbonate in 1,4-dioxane;water at 100; for 2 h;Inert atmosphere;
Steps:
III-58.2
5-Bromo-pyridin-3-ylamine (1.0 g, 5.8 mmol), bis(pinacolato)diboron (1.46 g, 5.7 mmol), Pd(dppf)Cl2 (200 mg), AcOK (1.1 g, 11.4 mmol) were stirred in 1,4-dioxane (15 mL) at 100°C under N2 for 4 h. After cooled to room temperature, to the mixture was added 2- bromo-pyridine (0.91 g, 5.7 mmol), Cs2C03 (7.4 g, 22.8 mmol), Pd(PPh3)4 (200 mg), water (3 mL). The mixture was then heated to 100 °C under N2 for 2 h. The solvent was concentrated under reduced pressure. The residue was dissolved in water and the aqueous phase was extracted with EtOAc (30 mL x3). The organic layer was washed with brine, dried over anhydrous Na2S04 and concentrated to dryness under reduced pressure. The crude was purified via silic gel column (DCM/MeOH, 20/1) to afford 300 mg (2-step yield: 30%) of [2,3*]Bipyridinyl-5*- ylamine. MS: m/z 172.0 (M+H+).
References:
WO2013/126608,2013,A1 Location in patent:Paragraph 00394
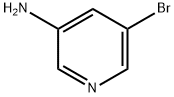
13535-01-8
387 suppliers
$3.00/1g

1245745-55-4
13 suppliers
inquiry