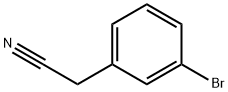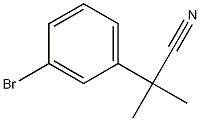
2-(3-Bromophenyl)-2-methylpropanenitrile synthesis
- Product Name:2-(3-Bromophenyl)-2-methylpropanenitrile
- CAS Number:90433-20-8
- Molecular formula:C10H10BrN
- Molecular Weight:224.1
Yield:90433-20-8 100%
Reaction Conditions:
Stage #1:(3-bromophenyl)acetonitrile with lithium hexamethyldisilazane in tetrahydrofuran at 0; for 0.333333 h;
Stage #2:methyl iodide in tetrahydrofuran at 20; for 1 h;
Steps:
2.1
Lithium bis(trimethylsilyl)amide (1 M in tetrahydrofuran, 58.7 ml, 58.7 mmol) was added slowly to a solution of 2-(3-bromophenyl)acetonitrile (5.75 g, 29.33 mmol) in tetrahydrofuran (60 ml) cooled down to 00C. After complete addition, the reaction mixture was stirred at 00C for a further 20 minutes. Methyl iodide (9.14 ml, 146.82 mmol) was then added to the reaction mixture and the reaction was stirred at room temperature for 1 hour. The crude mixture was quenched with water and extracted with ethyl acetate. The organic phase was washed with brine, dried over magnesium sulfate, filtered and concentrated in vacuo. The residue was purified on silica gel by flash column chromatography to afford the title compound as a colourless oil (6.629 g, quantitative yield).1H NMR (MeOH-d4, 400 MHz) δ 1.57 (6H, s), 7.19 (IH, t), 7.33-7.38 (2H, m), 7.53 (IH, t); MS (ES+) 225.
References:
VERTEX PHARMACEUTICALS INCORPORATED WO2009/73300, 2009, A1 Location in patent:Page/Page column 107