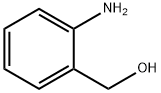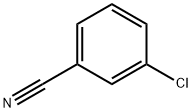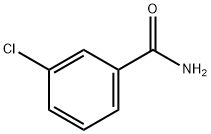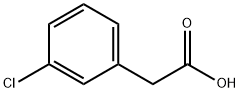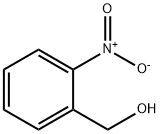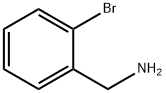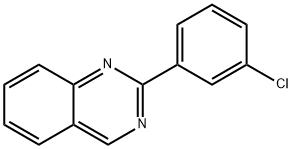
2-(3-chlorophenyl)quinazoline synthesis
- Product Name:2-(3-chlorophenyl)quinazoline
- CAS Number:1353000-31-3
- Molecular formula:C14H9ClN2
- Molecular Weight:240.69
501446-99-7
0 suppliers
inquiry

1353000-31-3
6 suppliers
inquiry
Yield:1353000-31-3 223 mg
Reaction Conditions:
with Trametes versicolor laccase (E.C. 1.10.3.2);oxygen;2,3-dicyano-5,6-dichloro-p-benzoquinone in aq. phosphate buffer at 45; pH=4.5; for 22 h;Enzymatic reaction;
Steps:
2.3. General procedure for the aerobic oxidative synthesis of2-substituted quinazolines by laccase/DDQ catalyst system
General procedure: A solution of 2-aminobenzylamine (1 mmol) and aldehydes(1 mmol) in 5 mL of CH3CN was stirred at room temperature until the cyclocondensation was completed (about 3-5 h). After complete conversion of 2-aminobenzylamine to tetrahydroquinazoline (TLC), the solvent was concentrated to 0.5 mL and DDQ (45.4 mg,0.2 mmol), and sodium phosphate buffer solution (NaPBS)(0.1 M, pH 4.5, 12.5 mL) and laccase from T. versicolor (100 U, 89 mg) were added. Then, the mixture was stirredat 45 °C under molecular oxygen (balloon) or air for the time given in Table 1. After completion of the reaction(monitored by TLC), the mixture was extracted by EtOAc(3 10 mL) and dried over anhydrous Na2SO4. The crudeproduct obtained after the removal of a solvent under vacuum was purified by chromatography on silica gel (nhexane/ethyl acetate, 3/1). All of the products were identified and characterized by comparing their spectral data(1H NMR) and physical properties (melting point) with those of authentic samples (see Supplementary data).
References:
Shariati, Mastaneh;Imanzadeh, Gholamhassan;Rostami, Amin;Ghoreishy, Nadya;Kheirjou, Somayyeh [Comptes Rendus Chimie,2019,vol. 22,# 4,p. 337 - 346]